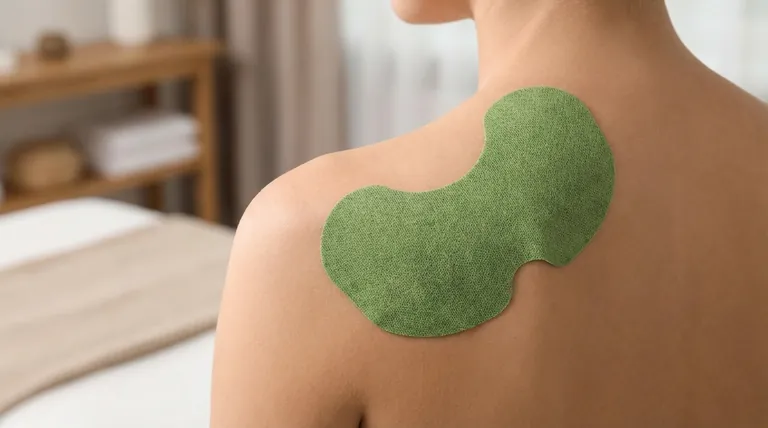
Belladonna plaster has evolved significantly from a traditional remedy into a modern, clinically proven medicinal product. Its current formulations are designed to deliver a standardized dose of active ingredients for the targeted relief of muscular aches, pains, and stiffness, with improved adhesive technology and safety profiles compared to historical versions.
The key takeaway is that modern Belladonna plaster is no longer a simple herbal patch. It is a regulated, evidence-based treatment whose formulation has been refined for consistent performance and safety in relieving localized pain.
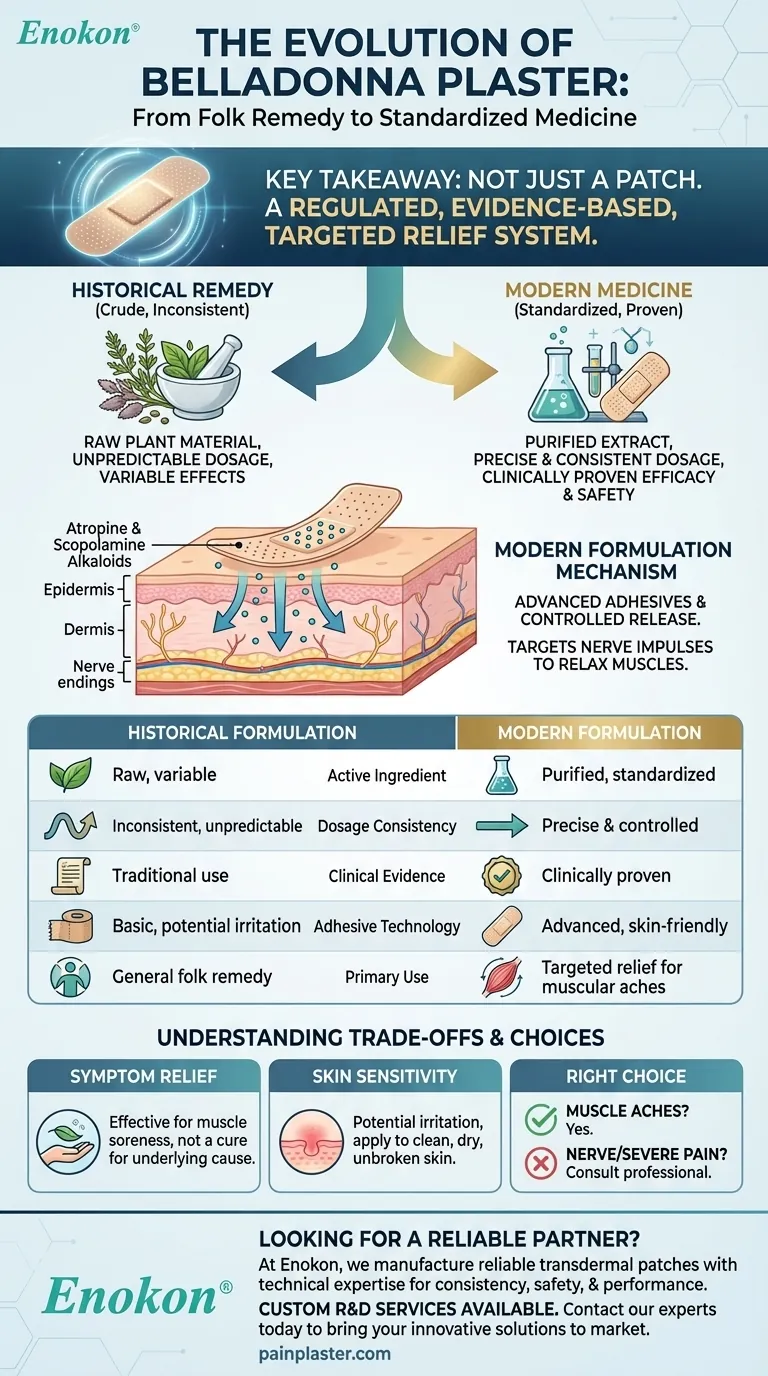
From Historical Remedy to Modern Medicine
The journey of the Belladonna plaster reflects the broader evolution of medicine itself—from reliance on raw botanicals to the precision of modern pharmaceuticals.
The Original Formulation's Challenge
Historically, plasters were made with raw or crudely processed Belladonna plants. This led to a significant problem: inconsistent dosage. The concentration of active alkaloids could vary wildly, making both the effects and the potential for side effects unpredictable.
The Shift to Standardization
The primary improvement in modern formulations is standardization. Manufacturers now use purified Belladonna extract, ensuring each plaster contains a precise, consistent amount of the active components. This guarantees a predictable therapeutic effect and enhances the product's safety.
The Introduction of Clinical Proof
The statement that it is "clinically proven" signifies a major shift. This means the modern product has undergone studies to validate its effectiveness and safety for its intended use. It has moved from the realm of folk medicine to that of an evidence-based therapeutic option.
Understanding the Modern Formulation
Today's Belladonna plaster is a carefully engineered drug delivery system. The improvements go beyond just the active ingredient.
The Role of Belladonna Extract
The plaster delivers alkaloids, primarily atropine and scopolamine, directly through the skin. These compounds work by blocking certain nerve impulses, which helps to relax tense muscles and interrupt pain signals at the source, providing localized relief.
Advanced Adhesives and Delivery
Modern formulations feature advanced, skin-friendly adhesives. These are designed to hold the plaster securely in place for extended periods while minimizing skin irritation. The plaster itself acts as a vehicle for the slow, controlled release of the medication over several hours.
Understanding the Trade-offs
Like any medical treatment, it's important to understand the context and limitations of using a Belladonna plaster.
Not a Cure, but a Symptom Reliever
The plaster is highly effective for managing symptoms like muscle soreness, stiffness, and localized aches. However, it does not treat the underlying cause of the pain.
Potential for Skin Irritation
While adhesives have improved, some individuals may still experience skin sensitivity or irritation. It is crucial to apply the plaster to clean, dry, and unbroken skin to minimize this risk.
The Importance of Correct Use
Because it contains potent active ingredients, the plaster must be used as directed. It should not be used on open wounds or mucous membranes, and the recommended application time should not be exceeded.
Making the Right Choice for Your Goal
To determine if a modern Belladonna plaster is the appropriate choice, consider your specific need.
- If your primary focus is targeted relief for muscle stiffness or aches: This product is an excellent, localized option that avoids the systemic effects of oral pain medication.
- If you have very sensitive skin or known allergies to adhesives: You should exercise caution, perform a small patch test first, or explore alternative pain relief methods.
- If you are dealing with nerve pain, widespread pain, or a severe injury: It is essential to consult a healthcare professional, as Belladonna plaster is designed for muscular pain and may not be the appropriate treatment.
Ultimately, recognizing the Belladonna plaster as a standardized medicinal product allows you to use it safely and effectively for its intended purpose.
Summary Table:
| Aspect | Historical Formulation | Modern Formulation |
|---|---|---|
| Active Ingredient | Raw, variable Belladonna plant | Purified, standardized extract |
| Dosage Consistency | Inconsistent and unpredictable | Precise and controlled |
| Clinical Evidence | Based on traditional use | Clinically proven efficacy & safety |
| Adhesive Technology | Basic, potential for irritation | Advanced, skin-friendly |
| Primary Use | General folk remedy | Targeted relief for muscular aches & stiffness |
Looking for a reliable partner for your transdermal patch development?
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands. Our technical expertise ensures your product, like a modern Belladonna plaster, meets the highest standards of consistency, safety, and performance.
Benefit from our custom R&D and development services to bring your innovative pain relief solutions to market.
Contact our experts today to discuss your project requirements.
Visual Guide
Related Products
- Natural Herbal Wormwood Patch Pain Plaster
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Medical Cooling Gel Patches for Fever Cooling Patches
- Cooling Fever Patches Color Change Cold Fever Patch
People Also Ask
- What precautions should be taken when using pain relief patches? Essential Safety Guide
- What medical conditions should be reported before using buprenorphine patches? Essential Safety Guide
- What are pain relief patches? Discover Targeted, Drug-Free Pain Management Solutions
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing
- Who should consult a healthcare professional before using pain relief patches? Ensure Your Safety with Medical Advice
















