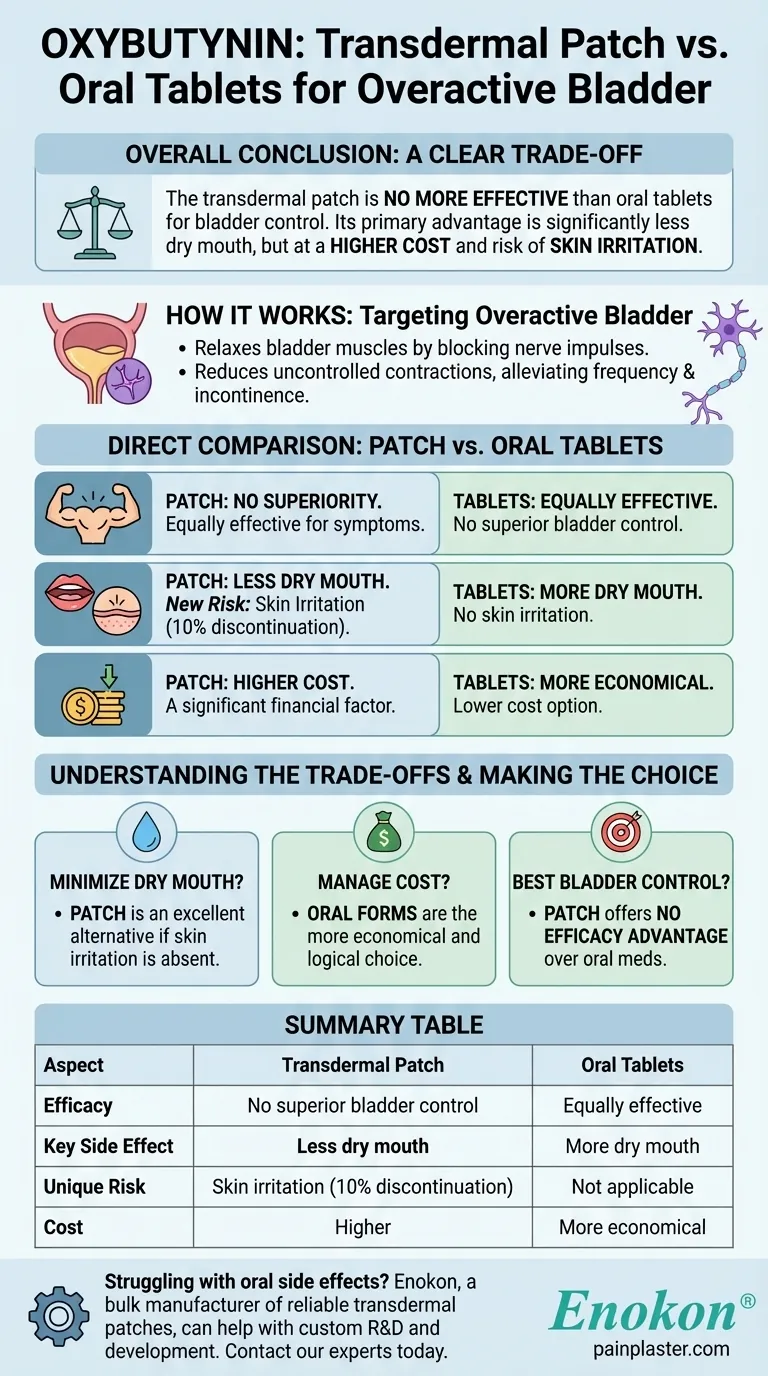
In short, the transdermal oxybutynin patch is no more effective than the oral tablet forms for treating an overactive bladder. Its primary advantage is causing significantly less dry mouth, a common side effect of the oral medication. However, this benefit comes at a higher financial cost and introduces the risk of skin irritation, which is severe enough to cause about 10% of patients to discontinue its use.
The central decision for using the oxybutynin patch is a clear trade-off. It does not offer superior bladder control but exchanges the common side effect of dry mouth for a higher cost and the potential for skin reactions.

How the Oxybutynin Patch Works
The Target: Overactive Bladder
The oxybutynin patch is designed to treat an overactive bladder. This is a condition where the bladder muscles contract uncontrollably.
These contractions lead to symptoms like urinary frequency, a sudden and urgent need to urinate, and incontinence (the inability to control urination).
The Mechanism: Relaxing Bladder Muscles
Oxybutynin belongs to a class of drugs called antimuscarinics. It works by blocking certain nerve impulses, which in turn helps relax the muscles of the bladder.
This relaxation reduces the uncontrolled contractions, thereby alleviating the primary symptoms of an overactive bladder. The patch delivers a continuous, steady dose of the medication through the skin.
A Direct Comparison: Patch vs. Oral Tablets
Efficacy: A Level Playing Field
The most critical point to understand is that the patch is not more effective than either the short-acting or long-acting oral versions of oxybutynin.
All forms of the medication work to control symptoms, but the transdermal route does not provide a superior level of bladder control.
Side Effects: The Key Differentiator
The main reason to choose the patch is its different side effect profile. By bypassing the digestive system, it causes significantly less dry mouth.
However, it introduces a new side effect: skin reactions at the application site. These reactions can include redness, itching, or a rash.
Cost: A Clear Disadvantage
The transdermal patch is consistently more expensive than its oral counterparts. This financial factor is a significant part of the decision-making process for many patients.
Understanding the Trade-offs
The Primary Benefit: Reduced Dry Mouth
For patients who find the dry mouth from oral oxybutynin to be intolerable, the patch presents a valuable alternative that may allow them to continue effective treatment.
The Primary Drawback: Skin Reactions
The key risk with the patch is local skin irritation. For about 10% of patients, this reaction is bothersome enough to make them stop using the patch altogether.
The Practical Considerations: Application
The patch is a 39 cm² matrix system containing 36 mg of oxybutynin, designed to deliver 3.9 mg per day. It should be applied to clean, dry skin on the abdomen, hip, or buttock.
To minimize skin irritation, it is crucial to rotate the application site with each new patch, which is typically replaced every 3 to 4 days. Adhesion is generally very good, with less than 1% of patches detaching.
Making the Right Choice for Your Goal
The decision between oral and transdermal oxybutynin hinges entirely on your personal priorities and tolerance for specific side effects.
- If your primary focus is minimizing dry mouth: The patch is an excellent alternative, provided you do not develop significant skin irritation.
- If your primary focus is managing cost: The oral forms are the more economical and logical choice.
- If your primary focus is achieving the best possible bladder control: The patch offers no efficacy advantage over the oral medications.
Ultimately, the oxybutynin patch provides a valuable alternative for a specific subset of patients, allowing for a more personalized approach to managing overactive bladder.
Summary Table:
| Aspect | Transdermal Patch | Oral Tablets |
|---|---|---|
| Efficacy | No superior bladder control | Equally effective |
| Key Side Effect | Less dry mouth | More dry mouth |
| Unique Risk | Skin irritation (10% discontinuation) | Not applicable |
| Cost | Higher | More economical |
Struggling with the side effects of oral overactive bladder medication?
Enokon, a bulk manufacturer of reliable transdermal patches, can help you develop a better solution. Our technical expertise in custom R&D and development is ideal for healthcare and pharma distributors and brands looking to create effective, patient-friendly transdermal products.
Contact our experts today to discuss how we can partner on your next transdermal project.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
People Also Ask
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
















