The risk of a blood clot from the contraceptive patch is very low for most people, affecting up to 1 in 1,000 individuals using this type of combined hormonal contraception. While rare, these events can be serious, including the formation of clots in the leg or lung, which can potentially lead to a heart attack or stroke.
While the baseline risk of blood clots from the patch is statistically small, this risk is not the same for everyone. It is a serious potential side effect that is significantly influenced by personal health factors like smoking, age, and cardiovascular history.

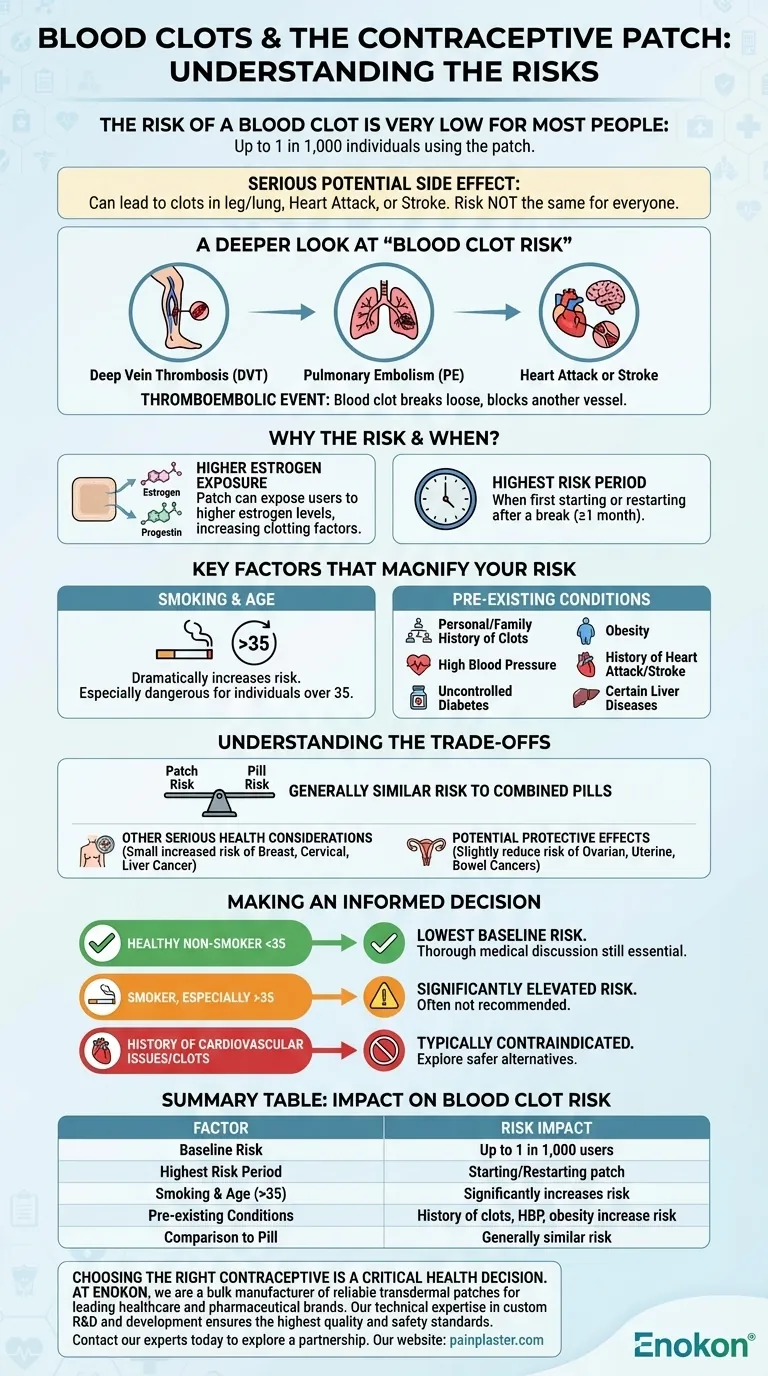
A Deeper Look at "Blood Clot Risk"
To make an informed decision, it's crucial to understand what the term "blood clot risk" truly entails and why it's associated with the patch.
What is a Thromboembolic Event?
This is the medical term for a blood clot that forms in a blood vessel, breaks loose, and is carried by the bloodstream to block another vessel.
With the patch, this can manifest as:
- Deep Vein Thrombosis (DVT): A clot forming in a deep vein, usually in the leg.
- Pulmonary Embolism (PE): A DVT that travels to the lungs, blocking a vital artery.
- Heart Attack or Stroke: A clot blocking blood flow to the heart or brain.
These events can cause permanent disability or, in rare cases, be fatal.
Why Does the Patch Increase This Risk?
The contraceptive patch is a form of combined hormonal contraception, meaning it releases both estrogen and progestin into your body.
The patch can expose users to higher levels of estrogen compared to some birth control pills. Estrogen is known to increase the concentration of clotting factors in the blood, which is the primary mechanism behind this elevated risk.
When is the Risk Highest?
The risk of developing a blood clot is not constant. It is highest when you first start using the patch or when you restart it after a break of a month or more.
Key Factors That Magnify Your Risk
Your individual health profile is the most important element in assessing your true risk. The baseline statistic of 1 in 1,000 does not apply equally to everyone.
The Impact of Smoking and Age
The combination of smoking and using hormonal contraception like the patch dramatically increases your risk of blood clots and cardiovascular events. This risk becomes especially pronounced for individuals over the age of 35.
Pre-existing Health Conditions
The patch is often not recommended for individuals with certain health conditions due to the increased risk. These include:
- A personal or family history of blood clots
- High blood pressure
- Uncontrolled diabetes
- Obesity (some patches carry a specific warning for this)
- History of heart attack or stroke
- Certain liver diseases
Understanding the Trade-offs
Choosing any medication involves weighing its effectiveness and benefits against its potential risks.
How the Patch Compares to the Pill
The risks associated with the patch are generally considered similar to those of combined oral contraceptive pills. Both methods use synthetic hormones and carry a comparable risk profile for blood clots and other serious side effects.
Other Serious Health Considerations
Beyond blood clots, long-term use of the patch is associated with a small increased risk of certain cancers, including breast, cervical, and liver cancer.
Potential Protective Effects
It is also important to note that, like other forms of combined hormonal contraception, the patch may slightly reduce the long-term risk of developing ovarian, uterine, and bowel cancers.
Making an Informed Decision
Your choice should be based on a clear understanding of your personal health profile in consultation with a healthcare provider.
- If you are a healthy non-smoker under 35: Your risk is at its lowest baseline, but a thorough discussion of your medical history with a doctor is still essential.
- If you smoke, especially if you are over 35: Your risk is significantly elevated, and this method is often not the recommended choice.
- If you have a history of cardiovascular issues or blood clots: Combined hormonal methods like the patch are typically contraindicated, and safer alternatives should be explored.
Understanding your personal risk factors is the key to choosing the safest and most effective contraception for you.
Summary Table:
| Factor | Impact on Blood Clot Risk |
|---|---|
| Baseline Risk | Up to 1 in 1,000 users |
| Highest Risk Period | When first starting or restarting the patch |
| Smoking & Age (>35) | Significantly increases risk |
| Pre-existing Conditions | History of clots, high blood pressure, obesity can increase risk |
| Comparison to Pill | Generally considered similar risk |
Choosing the right contraceptive is a critical health decision. At Enokon, we are a bulk manufacturer of reliable transdermal patches for leading healthcare and pharmaceutical brands. Our technical expertise in custom R&D and development ensures the highest quality and safety standards for your products. Let's discuss how we can support your brand with innovative, effective transdermal solutions. Contact our experts today to explore a partnership.
Visual Guide
Related Products
- Prostate Pain Kidney Health Care Patch for Men
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Menthol Gel Pain Relief Patch
People Also Ask
- What should be done if a testosterone patch falls off? A Guide to Maintaining Hormone Stability
- What should be done in case of a testosterone patch overdose? A Step-by-Step Emergency Guide
- What should be done if a dose of testosterone patches is missed? Regain Stability and Safety
- What should be done if a testosterone patch is missed or falls off? Follow these simple timing rules for safety and consistency.
- What should patients tell their doctor before using testosterone patches? A Guide to Safe Treatment















