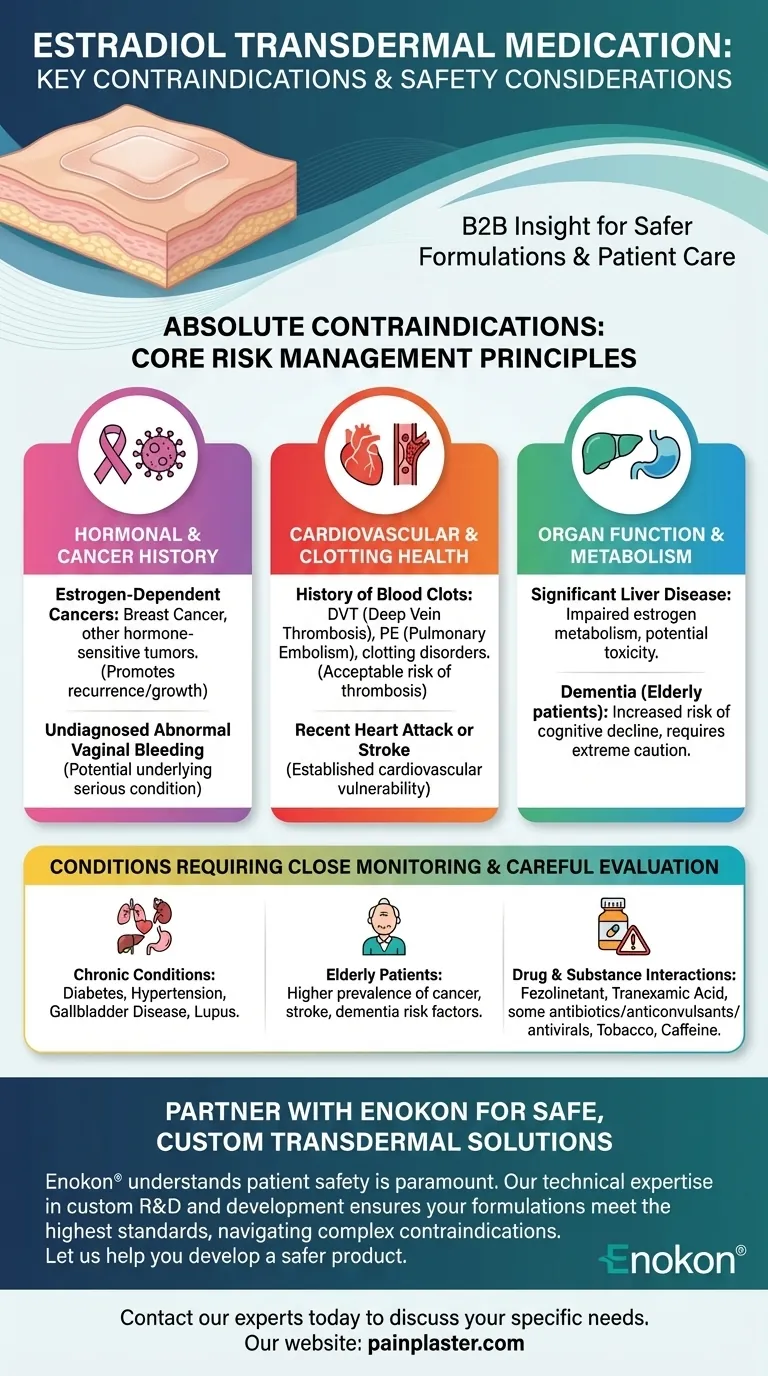
Certain medical histories absolutely contraindicate the use of estradiol transdermal medication due to the risk of severe adverse events. These conditions primarily include a history of estrogen-dependent cancers like breast cancer, a history of blood clots, recent heart attack or stroke, significant liver disease, and undiagnosed abnormal vaginal bleeding.
The core principle behind these contraindications is risk management. Estradiol is a powerful hormone that can promote cell growth and affect blood clotting, making its use unsafe for individuals with pre-existing conditions that could be dangerously exacerbated by these effects.

Why Hormonal and Cancer History Matters
The primary concern with estrogen therapy is its potential to stimulate the growth of certain hormone-sensitive tissues.
Estrogen-Dependent Cancers
Estradiol can act as a fuel for cancers that have estrogen receptors, such as certain types of breast cancer or other estrogen-dependent tumors.
Administering estradiol to someone with a history of these cancers could promote a recurrence or accelerate growth.
Undiagnosed Vaginal Bleeding
Abnormal vaginal bleeding is a potential symptom of underlying conditions like endometrial hyperplasia or cancer.
Using estradiol before diagnosing the cause of the bleeding could worsen a serious underlying condition. It is a critical warning sign that must be investigated first.
The Critical Role of Cardiovascular and Clotting Health
Estradiol can influence the body's clotting mechanisms and cardiovascular system, increasing risk for individuals with a compromised history.
History of Blood Clots
Estrogen therapy is known to increase the risk of developing blood clots in the legs (deep vein thrombosis) or lungs (pulmonary embolism).
For individuals with a personal history of blood clots or a known blood clotting disorder (thrombophilia), this risk is considered unacceptable.
Recent Heart Attack or Stroke
Patients with a recent heart attack or stroke have an established vulnerability in their cardiovascular system.
The increased risk of clot formation associated with estradiol makes it a dangerous choice for these individuals.
Organ Function and Cognitive Health Concerns
The body's ability to process hormones and potential cognitive side effects are also key considerations.
Significant Liver Disease
The liver is responsible for metabolizing and breaking down estrogen.
If liver function is impaired, estradiol levels can build up in the body, leading to toxicity and an increased risk of side effects.
Dementia
While not an absolute contraindication in all cases, studies have shown that estrogen therapy may increase the risk of dementia in postmenopausal women.
Therefore, its use requires extreme caution in elderly patients or those with existing cognitive decline.
Understanding the Trade-offs and Cautions
Beyond absolute contraindications, several conditions require careful evaluation and monitoring because they can be negatively impacted by estradiol.
Conditions Requiring Close Monitoring
Chronic conditions like diabetes, hypertension, gallbladder disease, and autoimmune disorders like lupus require a careful risk-benefit analysis with a healthcare provider.
These conditions can be influenced by hormonal changes, fluid retention, or other effects of estradiol.
Considerations for Elderly Patients
Elderly patients are more likely to have underlying conditions like breast cancer, stroke, or dementia.
While age itself is not a contraindication, the higher prevalence of these risk factors necessitates heightened caution and thorough screening.
Key Drug and Substance Interactions
Estradiol can interact with many other substances. Its use is not recommended with fezolinetant or tranexamic acid.
Dose adjustments may also be needed when taken with certain antibiotics, anticonvulsants, and antivirals. Interactions can also occur with tobacco and caffeine.
How to Apply This to Your Health
A transparent and thorough discussion with your doctor is the only way to determine if estradiol transdermal medication is safe for you.
- If your primary focus is safety with a cancer history: You must ensure any past or present cancers are not estrogen-sensitive before even considering this therapy.
- If your primary focus is cardiovascular health: You need to discuss your personal and family history of blood clots, stroke, or heart attacks to assess the thrombotic risk.
- If your primary focus is managing other chronic illnesses: The conversation will be about how estradiol might impact your specific condition, such as liver function, blood pressure, or blood sugar control.
Ultimately, your complete medical history is the critical document that will guide a safe and informed decision with your healthcare provider.
Summary Table:
| Contraindication Category | Specific Conditions | Primary Concern |
|---|---|---|
| Cancer History | Breast cancer, other estrogen-dependent tumors | Promotes cancer recurrence/growth |
| Cardiovascular/Clotting | History of blood clots (DVT/PE), recent heart attack/stroke | Increases risk of thrombosis |
| Organ Function | Significant liver disease, undiagnosed vaginal bleeding | Toxicity from impaired metabolism; masks serious conditions |
| Neurological | Dementia (in elderly patients) | May increase risk of cognitive decline |
Partner with Enokon for Safe, Custom Transdermal Solutions
As a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands, Enokon understands that patient safety is paramount. Our technical expertise in custom R&D and development ensures your formulations meet the highest standards, helping you navigate complex contraindications.
Let us help you develop a safer product. Contact our experts today to discuss your specific needs.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Pain Patch Relief Pain Reliever for Back
People Also Ask
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief














