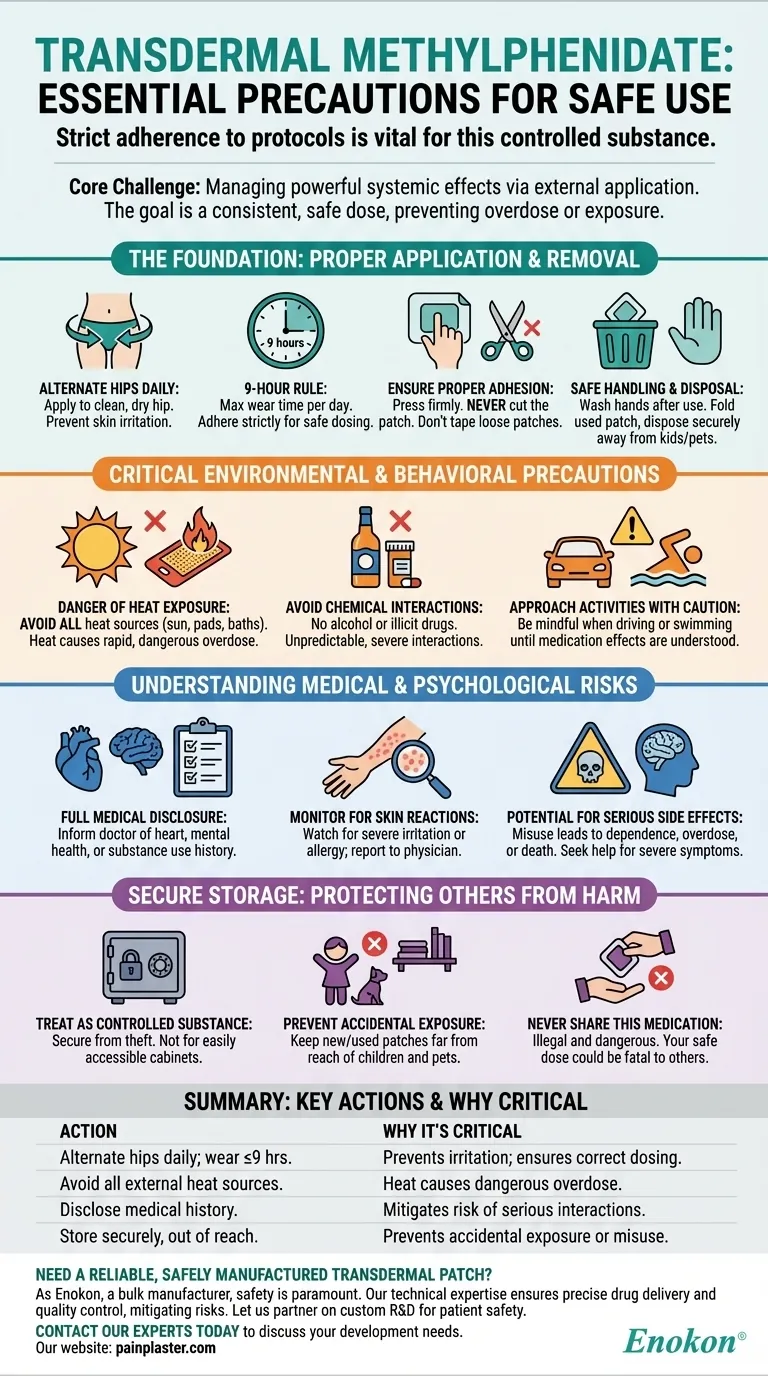
Using a transdermal methylphenidate patch requires strict adherence to specific safety protocols. This medication is a controlled substance that demands careful handling, precise application, and an understanding of potential risks. Key precautions involve applying the patch to a new, clean hip area each day for no more than nine hours, avoiding all external heat sources, and storing it securely to prevent misuse or accidental exposure.
The core challenge with a transdermal patch is managing its powerful systemic effects through a simple external application. Your primary responsibility is to ensure a consistent, safe dose for the user while rigorously preventing accidental overdose or exposure to others.
The Foundation: Proper Application and Removal
Correct application is the first line of defense in ensuring both the efficacy and safety of transdermal methylphenidate. Errors at this stage can lead to incorrect dosing or skin complications.
Choosing the Right Application Site
Apply the patch to the hip area on clean, dry, and unbroken skin. It is crucial to alternate hips each day to prevent significant skin irritation.
The 9-Hour Rule
The patch should not be worn for more than nine hours. Adhering to this time limit is essential for managing the medication's dose and duration of effect.
Ensuring Proper Adhesion
Press the patch firmly onto the skin to ensure it is secure. Never cut the patch, as this can damage the medication-delivery system and lead to an unpredictable dose. If the patch loosens, do not attempt to fix it with tape or other adhesives.
Safe Handling and Disposal
Always wash your hands immediately after applying a patch. When a used patch is removed, fold it in half so the sticky sides press together, and dispose of it in a way that is inaccessible to children or pets.
Critical Environmental and Behavioral Precautions
Your actions and environment while wearing the patch can significantly alter how the medication is absorbed, creating potentially dangerous situations.
The Danger of Heat Exposure
Avoid all external heat sources near the patch. This includes heating pads, electric blankets, direct sunlight, or prolonged hot baths. Heat dramatically increases the rate at which the medication is absorbed into the bloodstream, which can lead to a sudden, dangerous overdose.
Avoiding Chemical Interactions
Refrain from using alcohol or illicit drugs while on this medication. These substances can cause unpredictable and severe interactions.
Activities to Approach with Caution
Avoid driving or operating heavy machinery until you understand how the medication affects you. You should also be mindful during activities like swimming or bathing that could cause the patch to loosen and fall off.
Understanding the Medical and Psychological Risks
Transdermal methylphenidate is a powerful stimulant with significant side effects. Full transparency with your healthcare provider is non-negotiable.
The Importance of Full Medical Disclosure
Before starting, you must inform your doctor about any personal or family history of heart problems, high blood pressure, seizures, or circulation issues. It is equally critical to disclose any history of mental health conditions like psychosis, tics, Tourette's syndrome, or substance use problems.
Monitoring for Skin Reactions
Pay close attention to the application site. Watch for signs of severe skin irritation or an allergic reaction, such as a rash, swelling, or blistering, and report them to your physician.
Potential for Serious Side Effects
Misuse of this medication can lead to physical dependence, substance use disorder, overdose, or even death. Be aware of serious symptoms like severe confusion, paranoia, seizures, or difficulty breathing, and seek immediate medical help if they occur.
Secure Storage: Protecting Others from Harm
As a Schedule II controlled substance, methylphenidate has a high potential for misuse and abuse. Protecting others from accidental exposure or intentional misuse is a critical responsibility.
Treating it as a Controlled Substance
Store the medication in a secure location that is protected from theft. This is not a medication to be left in an easily accessible medicine cabinet.
Preventing Accidental Exposure
Always keep the medication, both new and used patches, far out of the reach and sight of children and pets. Even a used patch contains enough residual medication to be harmful.
Why You Must Never Share This Medication
It is illegal and extremely dangerous to share this prescription with anyone else. A dose that is safe for you could be harmful or fatal to another person.
Key Priorities for Safe Medication Use
Your approach to using transdermal methylphenidate should be guided by your primary safety concerns.
- If your primary focus is consistent treatment: Prioritize alternating application sites daily and following the strict 9-hour wear time.
- If your primary focus is preventing accidental overdose: Your most critical precaution is to avoid all external heat sources near the patch.
- If your primary focus is the safety of your household: Ensure the medication is stored securely and used patches are disposed of immediately and correctly.
By integrating these precautions into your routine, you can effectively manage treatment while minimizing the inherent risks of this medication.
Summary Table:
| Precaution Category | Key Action | Why It's Critical |
|---|---|---|
| Application & Removal | Alternate hips daily; wear for ≤9 hours. | Prevents skin irritation & ensures correct dosing. |
| Environmental Safety | Avoid all external heat sources (sun, heating pads). | Heat can cause a dangerous overdose via rapid absorption. |
| Medical Disclosure | Disclose heart, mental health, or substance use history. | Mitigates risk of serious side effects or interactions. |
| Secure Storage | Store new/used patches securely, out of reach of children/pets. | Prevents accidental exposure or misuse of the controlled substance. |
Need a reliable, safely manufactured transdermal patch?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands, we understand that safety is paramount. Our technical expertise ensures precise drug delivery systems and rigorous quality control, mitigating the risks associated with transdermal medications.
Let us partner with you on custom R&D to develop patches that prioritize patient safety and treatment efficacy.
Contact our experts today to discuss your transdermal patch development needs.
Visual Guide
Related Products
- Natural Herbal Wormwood Patch Pain Plaster
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Heat Pain Relief Patches Transdermal Patches
People Also Ask
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management
- What medical conditions should be reported before using buprenorphine patches? Essential Safety Guide
- What was the reported pain relief after the initial month of plaster use? Consistent & Effective Pain Management
- What are pain relief patches? Discover Targeted, Drug-Free Pain Management Solutions
- When should the pain relief patch not be used? Key Safety Rules to Avoid Risks

















