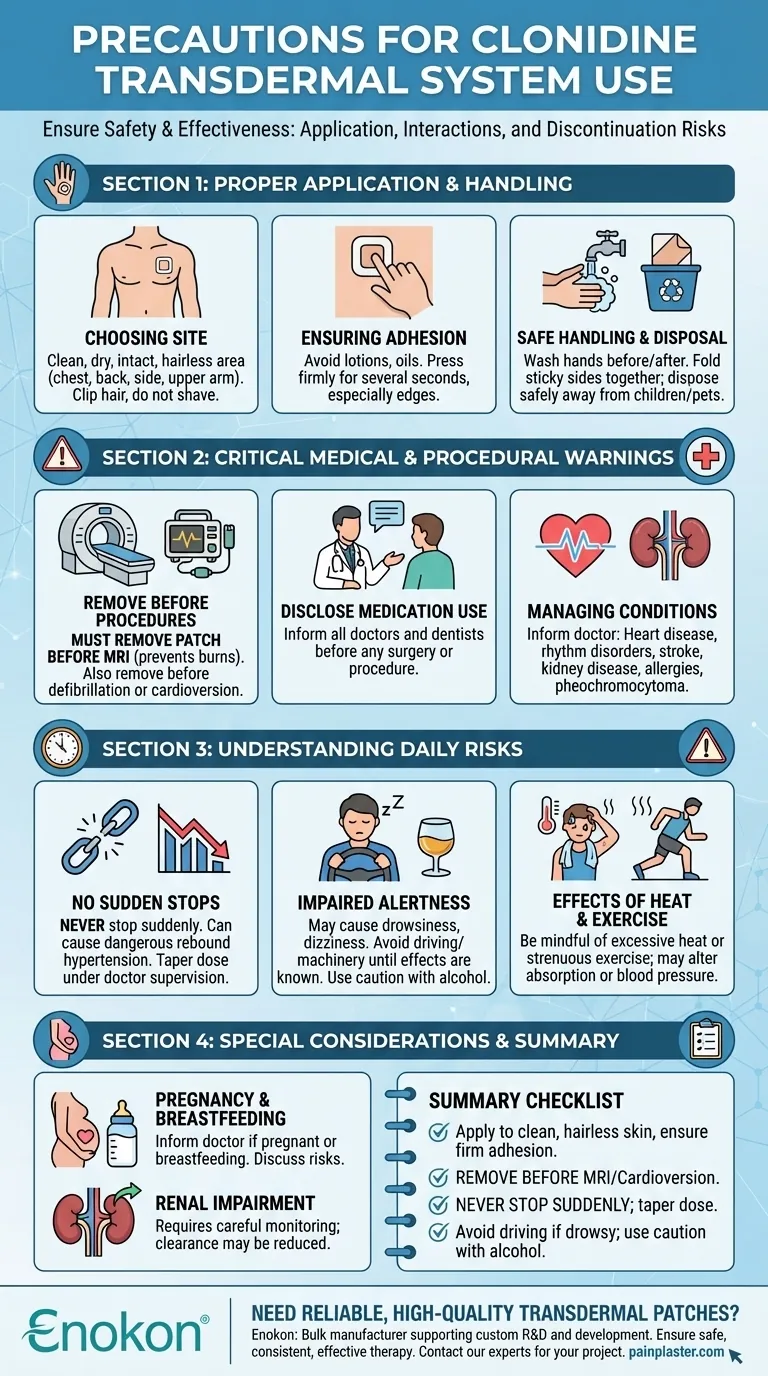
To ensure safety and effectiveness, using the clonidine transdermal system requires strict adherence to precautions related to patch application, awareness of critical medical interactions, and understanding the significant risks of abruptly stopping the medication. You must remove the patch before an MRI to prevent burns and always consult your doctor before discontinuing use to avoid a dangerous spike in blood pressure.
The most critical precaution with the clonidine patch is not its daily use, but its discontinuation. Never stop using it suddenly. Safe and effective therapy depends on proper application, awareness of procedural risks like MRIs, and a medically supervised plan to taper off the medication.

Proper Application and Handling: The Foundation of Safety
Correctly applying and handling the patch is the first step in ensuring consistent medication delivery and minimizing local side effects.
Choosing the Right Application Site
Apply the patch to a clean, dry, and intact area of skin. The best locations are flat, hairless parts of the body like the chest, back, side, or the outer part of the upper arm.
If the area has hair, clip it short but do not shave it, as shaving can irritate the skin.
Ensuring Proper Adhesion
Avoid using lotions, oils, or other skin products on the area where you will apply the patch, as they can prevent it from sticking properly.
After applying the patch, press down firmly with the palm of your hand for several seconds, paying special attention to the edges to ensure a tight seal.
Safe Handling and Disposal
Always wash your hands with soap and water before and after handling a patch.
When you remove a used patch, fold it in half with the sticky sides together. Dispose of it carefully where children or pets cannot access it.
Critical Medical and Procedural Warnings
Clonidine can interact significantly with certain medical conditions and procedures. Full disclosure to your healthcare providers is essential.
Removing the Patch for Medical Procedures
The patch contains aluminum in its backing. This requires you to remove the patch before an MRI to prevent the risk of skin burns at the patch site.
It should also be removed prior to defibrillation or cardioversion procedures.
Disclosing Your Medication Use
You must inform all doctors and dentists that you are using the clonidine transdermal system before any surgery or procedure. While therapy is often continued during surgery, your medical team needs this information to manage your care safely.
Managing Pre-existing Conditions
Inform your doctor if you have a history of heart disease, severe coronary artery disease, heart rhythm disorders (like sinus node dysfunction or AV block), or a past heart attack or stroke.
Use also requires careful monitoring in patients with kidney disease, a history of allergic reactions to clonidine, or a pheochromocytoma (a tumor of the adrenal gland).
Understanding the Daily Precautions and Risks
Beyond application, daily life with the clonidine patch requires awareness of potential side effects and interactions.
The Critical Risk of Abrupt Discontinuation
Never stop using clonidine suddenly. Doing so can cause a rapid and dangerous increase in your blood pressure, a condition known as rebound hypertension.
Always keep an adequate supply of patches to ensure continuous therapy. Discontinuation must be done gradually by tapering the dose under a doctor's supervision.
Impaired Alertness and Alcohol Interaction
Clonidine can cause drowsiness, dizziness, and may impair your thinking or reaction times. Avoid driving or operating heavy machinery until you know how it affects you.
Consuming alcohol can increase these side effects and should be done with caution.
Effects of Heat and Exercise
Be mindful of situations that increase your body temperature, such as excessive heat or strenuous exercise. These can potentially alter the absorption of the medication or affect your blood pressure.
Special Considerations
Certain populations must take additional precautions when using the clonidine transdermal system.
Use During Pregnancy and Breastfeeding
It is not definitively known if clonidine will harm an unborn baby. Inform your doctor if you are pregnant or planning to become pregnant.
Clonidine can pass into breast milk and may affect a nursing infant. Discuss the risks with your doctor if you are breastfeeding.
Considerations for Renal Impairment
Patients with kidney disease or impaired renal function require careful monitoring by a physician, as the body's ability to process and clear the medication may be reduced.
Making the Right Choice for Your Goal
Your specific situation dictates which precautions are most critical to you at any given time.
- If your primary focus is preparing for a medical procedure: You must inform your entire medical team and explicitly ask if the patch should be removed for an MRI or cardioversion.
- If your primary focus is managing daily activities: You must avoid driving or operating heavy machinery until you are certain how the medication affects your alertness.
- If your primary focus is changing your treatment: You must never stop abruptly; a gradual taper supervised by your doctor is essential to prevent a dangerous spike in blood pressure.
Understanding these precautions is the key to using the clonidine transdermal system safely and effectively as a partner in your healthcare.
Summary Table:
| Precaution Category | Key Actions |
|---|---|
| Application & Handling | Apply to clean, dry, hairless skin; ensure firm adhesion; wash hands before/after handling. |
| Medical Procedures | Remove patch before MRI or cardioversion; inform all doctors/dentists before surgery. |
| Discontinuation Risk | Never stop suddenly; taper dose under doctor supervision to avoid rebound hypertension. |
| Daily Activities | Can cause drowsiness; avoid driving/operating machinery until effects are known; use caution with alcohol. |
Need a reliable, high-quality transdermal patch for your healthcare or pharmaceutical brand?
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters. Our technical expertise supports custom R&D and development to meet your specific formulation and delivery needs.
Benefit from our experience: Ensure your patients receive safe, consistent, and effective transdermal therapy with patches manufactured to the highest standards.
Contact our experts today to discuss your custom transdermal patch development project.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief















