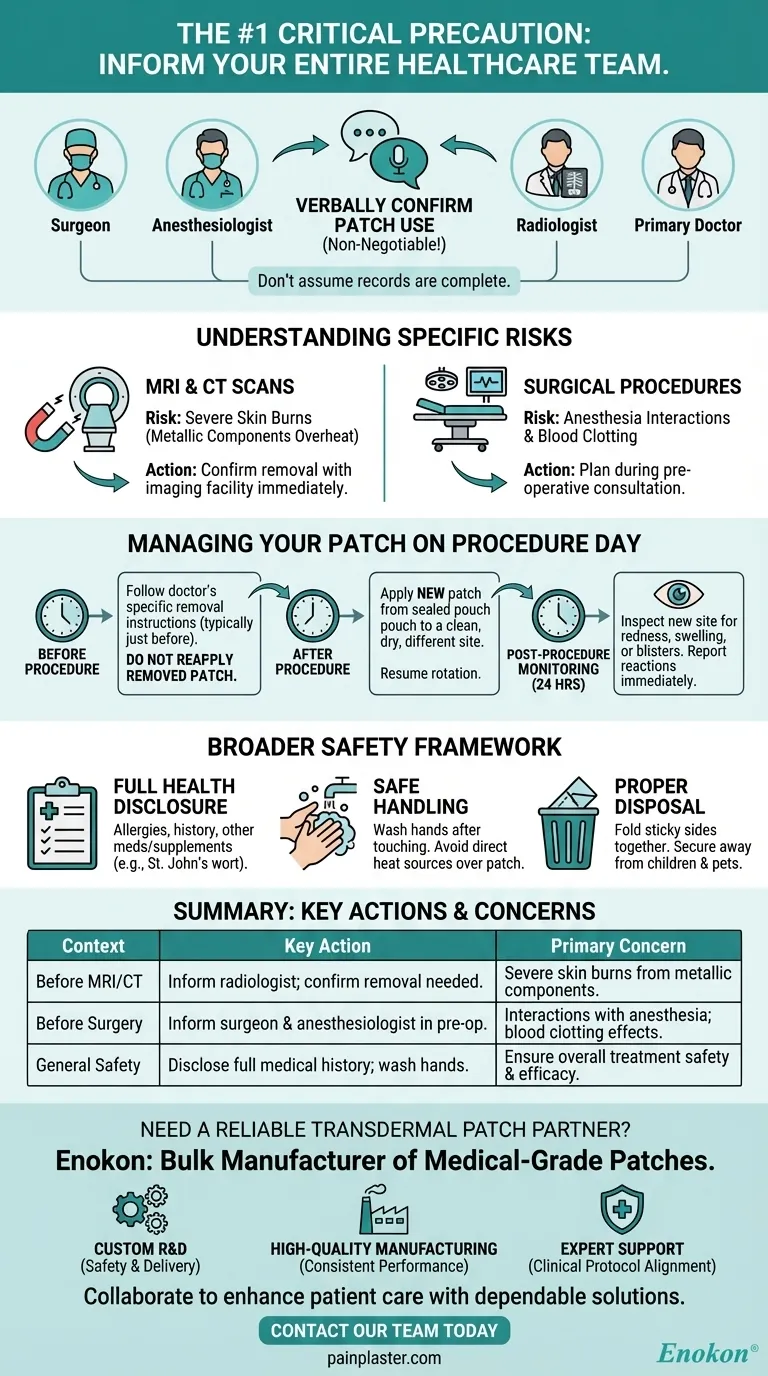
Before any medical procedure, the most critical precaution is to inform every member of your healthcare team—including your surgeon, anesthesiologist, and radiologist—that you are using an estradiol patch. Specific procedures, particularly MRIs, may require you to remove the patch temporarily to prevent serious skin burns.
The core issue is that some transdermal patches contain metallic components that can overheat during an MRI, posing a burn risk. For surgery, the concern shifts to potential interactions with anesthesia and effects on blood clotting, making proactive communication with your medical team non-negotiable for your safety.

Why Full Disclosure is Non-Negotiable
Failing to inform your medical team about your patch is not a minor oversight; it can have significant safety implications. Different procedures present different risks.
For MRI and CT Scans
The primary danger during an MRI is the potential for severe skin burns. Some patches contain aluminum or other metallic foils in their backing, which can heat up intensely when exposed to the powerful magnetic fields of an MRI machine. Always confirm with the imaging facility if removal is necessary.
For Surgical Procedures
During surgery, estradiol can potentially influence blood clotting and may interact with anesthetic agents. Your surgeon and anesthesiologist must have a complete picture of all medications you are taking to manage these risks effectively and ensure a safe procedure.
Who You Need to Tell
Do not assume your records are complete or have been reviewed by everyone. Verbally confirm your patch use with:
- Your surgeon and anesthesiologist during your pre-operative consultation.
- The radiologist or MRI technician just before your imaging scan.
- Your primary doctor or the specialist who prescribed the estradiol patch.
Managing Your Patch on Procedure Day
If your medical team advises you to remove the patch, you must also know how to manage it correctly to maintain therapeutic consistency.
Instructions for Removal and Reapplication
Your doctor will provide specific instructions. Typically, you will be told to remove the patch immediately before the procedure and apply a new one immediately after.
Do not try to reapply a patch that has been removed. Always use a new one from a sealed pouch.
Choosing a New Application Site
When you apply the new patch post-procedure, place it on a clean, dry, and non-irritated area of skin. Continue your normal site rotation schedule, avoiding the location of the previous patch to prevent skin irritation.
Post-Procedure Monitoring
After the procedure, inspect the new patch site over the next 24 hours. Report any unusual skin reactions, such as redness, swelling, or blisters, to your doctor immediately.
Understanding the Broader Safety Context
While procedure-specific precautions are vital, they are part of a larger framework for using estradiol patches safely.
Disclosing Your Full Health Profile
Your doctor must be aware of your complete medical history. This includes any allergies (especially to salicylates), history of cancer, blood disorders, asthma, or liver disease. Also disclose all other products you use, including vitamins and supplements like St. John's wort, which can affect how estradiol works.
Safe Handling and Application
Proper daily use is key to safety and efficacy. Always wash your hands after touching a patch, avoid applying heat (like from a heating pad) over it, and never apply a patch to broken or irritated skin.
Proper Disposal
Used patches still contain active medication. To dispose of one, fold it in half with the sticky sides together and place it in a trash receptacle that is out of reach of children and pets.
Making the Right Choice for Your Procedure
Your specific actions will depend on the type of medical intervention you are undergoing.
- If your primary focus is an upcoming MRI: Immediately contact the imaging facility and your prescribing doctor to confirm if your patch brand contains metal and requires removal.
- If your primary focus is preparing for surgery: Schedule a specific discussion with your surgeon and anesthesiologist to create a clear plan for managing your patch before, during, and after the operation.
- If your primary focus is any other medical test: Always err on the side of caution and inform the technician or doctor that you are wearing a transdermal patch.
Proactive and clear communication with your entire healthcare team is the key to safely navigating any medical procedure while on estradiol therapy.
Summary Table:
| Precautions | Key Actions | Primary Concern |
|---|---|---|
| Before MRI/CT Scan | Inform radiologist; confirm if patch removal is needed. | Severe skin burns from metallic components. |
| Before Surgery | Inform surgeon & anesthesiologist during pre-op consult. | Interactions with anesthesia; effects on blood clotting. |
| General Safety | Disclose full medical history; wash hands after handling. | Ensure overall treatment safety and efficacy. |
Need a reliable, safe transdermal patch for your patients?
As Enokon, a bulk manufacturer of medical-grade transdermal patches and pain plasters, we understand the critical importance of patient safety during medical procedures. Our patches are developed with a focus on quality and reliability, backed by extensive technical expertise.
We partner with healthcare distributors and pharmaceutical brands to provide:
- Custom R&D to meet specific safety and delivery requirements.
- High-quality manufacturing ensuring consistent performance.
- Expert support for developing patches that align with clinical safety protocols.
Let's collaborate to enhance patient care with dependable transdermal solutions. Contact our team today to discuss your needs.
Visual Guide
Related Products
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Menthol Gel Pain Relief Patch
People Also Ask
- Can pregnant women use pain relief patches? Your Essential Guide to Safe Pain Management
- Are natural and herbal pain relief patches effective and safe? Discover the Benefits of Targeted Relief
- Can the pain relief patch be used with other external analgesic products? A Critical Safety Guide
- What side effects might occur from using capsaicin patches? Understand the difference between normal sensation and danger signs.
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing














