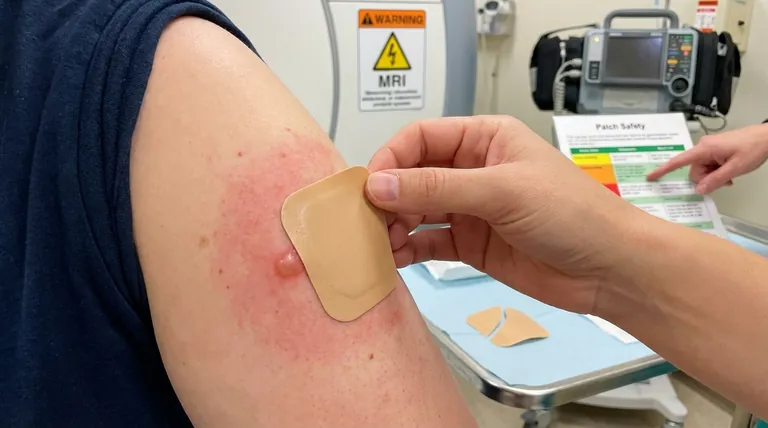
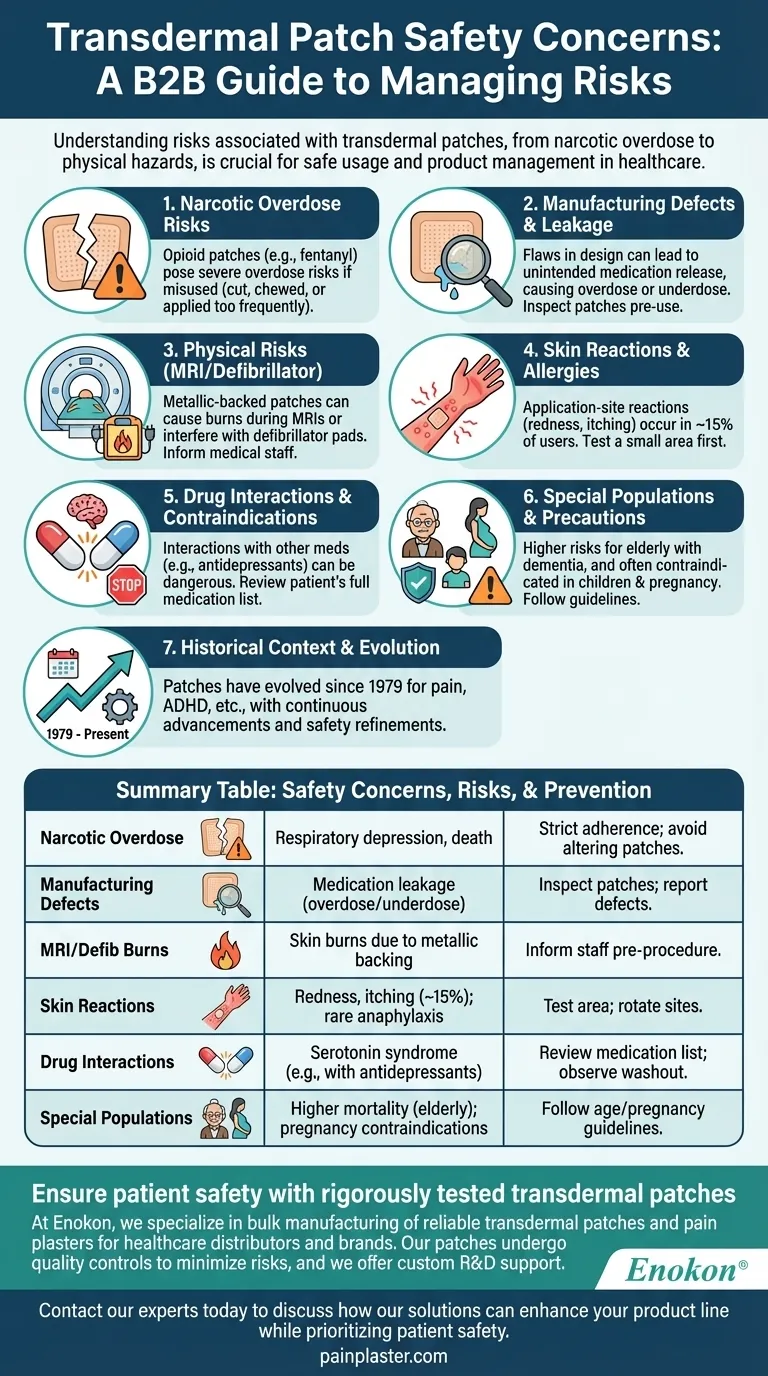
Transdermal patches, while convenient and effective for delivering medications, come with several safety concerns that users and healthcare providers must be aware of. These include risks of narcotic overdose, manufacturing defects leading to medication leakage, and physical risks like burns during MRIs or defibrillator use. Additionally, improper use—such as cutting, chewing, or changing patches too frequently—can lead to severe health consequences. Skin reactions and interactions with other medications further complicate their safety profile. Understanding these risks is crucial for ensuring safe usage and minimizing adverse effects.
Key Points Explained:
-
Narcotic Overdose Risks
- Transdermal patches containing opioids like fentanyl pose significant overdose risks if misused.
- Overdose can occur if patches are cut, chewed, or applied more frequently than prescribed.
- Symptoms may include severe respiratory depression, requiring immediate medical attention.
- Patients must strictly follow dosing instructions to avoid accidental overdose.
-
Manufacturing Defects and Medication Leakage
- Defects in patch design or production can lead to unintended medication release.
- Leakage increases the risk of overdose or underdose, compromising treatment efficacy.
- Users should inspect patches before application and report any defects to healthcare providers.
-
Physical Risks During Medical Procedures
- Metallic-backed patches can cause burns during MRI scans due to heat induction.
- Patches may interfere with defibrillator pads, leading to skin burns or ineffective treatment.
- Patients should inform medical staff about patch use before undergoing procedures.
-
Skin Reactions and Allergies
- Application-site reactions (e.g., redness, itching, blistering) occur in ~15% of users.
- Severe allergic reactions, though rare, can include anaphylaxis or angioedema.
- Testing a small area before full application can help identify sensitivities.
-
Drug Interactions and Contraindications
- Some patches (e.g., selegiline) interact dangerously with antidepressants, risking serotonin syndrome.
- A washout period (1–5 weeks) may be required when switching medications.
- Healthcare providers must review a patient’s full medication list before prescribing patches.
-
Special Populations and Usage Precautions
- Elderly patients with dementia face higher mortality risks when using antipsychotic patches.
- Patches are often contraindicated in children and pregnant women (e.g., pregnancy category C).
- Adherence to dietary restrictions (e.g., tyramine with selegiline) may be necessary for some patches.
-
Historical Context and Evolution
- Since the first FDA-approved patch in 1979, innovations have expanded their use for conditions like pain and ADHD.
- New patches are approved every ~2.2 years, reflecting ongoing advancements and safety refinements.
By addressing these concerns proactively—through proper education, adherence to guidelines, and vigilance during medical procedures—the risks associated with transdermal patches can be effectively managed. Have you considered how these safety measures might integrate into your current healthcare practices?
Summary Table:
| Safety Concern | Key Risks | Prevention Tips |
|---|---|---|
| Narcotic Overdose | Respiratory depression, death from misuse (cutting/chewing patches) | Strict adherence to dosing; avoid altering patches |
| Manufacturing Defects | Medication leakage leading to overdose/underdose | Inspect patches before use; report defects promptly |
| MRI/Defibrillator Burns | Skin burns due to metallic backing | Inform medical staff about patch use pre-procedure |
| Skin Reactions | Redness, itching, blistering (~15% of users); rare anaphylaxis | Test small area first; rotate application sites |
| Drug Interactions | Serotonin syndrome (e.g., selegiline + antidepressants) | Review full medication list; observe washout periods |
| Special Populations | Higher mortality in elderly with dementia; contraindications in pregnancy | Follow age/pregnancy guidelines; monitor high-risk patients closely |
Ensure patient safety with rigorously tested transdermal patches
At Enokon, we specialize in bulk manufacturing of reliable transdermal patches and pain plasters for healthcare distributors and brands. Our patches undergo stringent quality controls to minimize risks like leakage or defects, and we offer custom R&D support to address specific safety or efficacy needs.
Contact our experts today to discuss how our solutions can enhance your product line while prioritizing patient safety.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
- Medical Cooling Gel Patches for Fever Cooling Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
People Also Ask
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
















