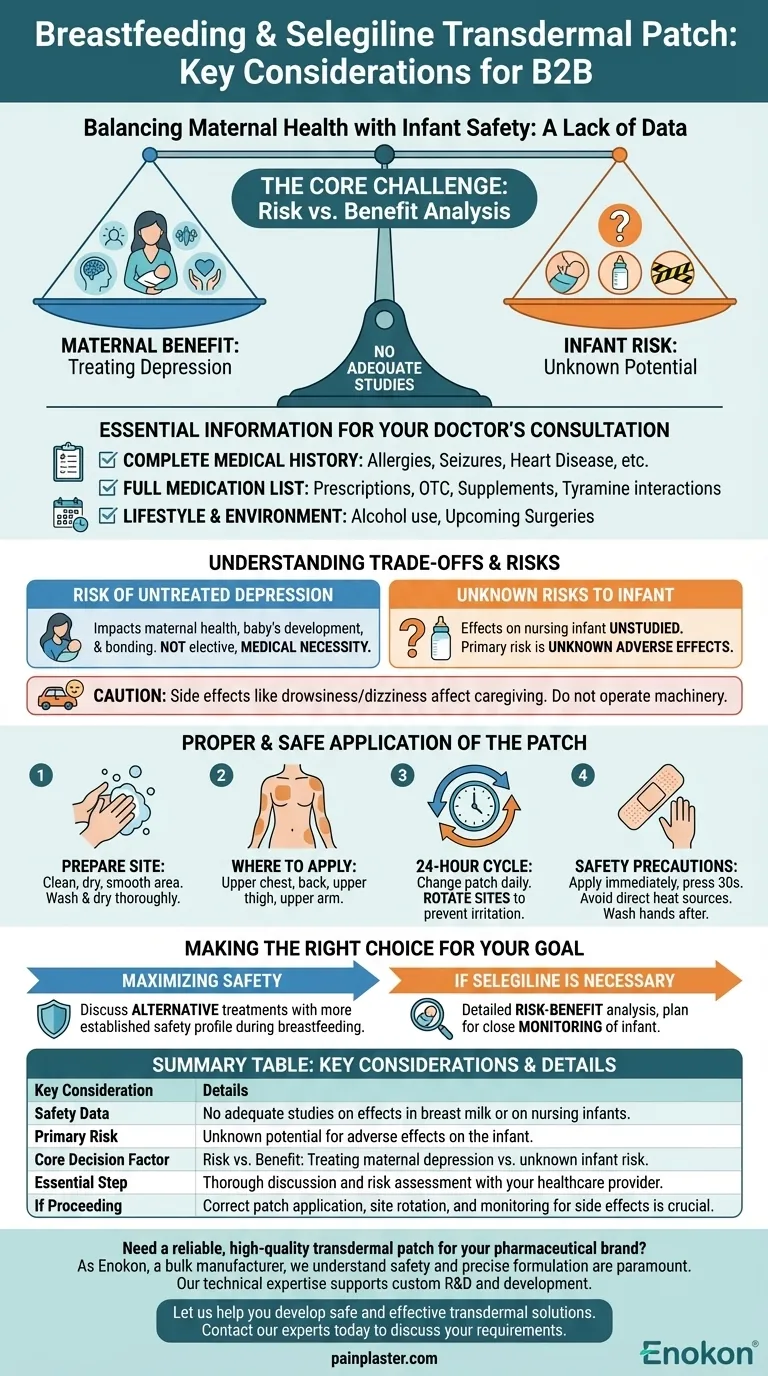
When considering the selegiline transdermal patch while breastfeeding, the most critical factor is the complete absence of adequate studies on its effects on a nursing infant. This lack of data means that any decision to use the medication requires a careful and thorough discussion with your doctor, weighing the significant benefits of treating your depression against the unknown potential risks to your child.
Due to a lack of safety data, the decision to use the selegiline patch while breastfeeding is a significant one. The central challenge is balancing the proven necessity of treating maternal depression against the unknown risks to the nursing child, a conversation that must be guided by your physician.

The Core Issue: A Lack of Safety Data
What the Research Says (and Doesn't Say)
There are currently no adequate clinical studies to determine the level of risk to an infant when the mother uses selegiline while breastfeeding. We do not know how much of the medication passes into breast milk or what its effects, if any, might be on a developing baby.
The Principle of Risk vs. Benefit
In situations with incomplete data, medical decisions hinge on a risk-versus-benefit analysis. The known, serious risks of untreated maternal depression must be weighed against the theoretical and unknown risks of the medication to the infant. Your health is a critical component of your baby's well-being.
Essential Information for Your Doctor's Consultation
To have the most productive conversation with your healthcare provider, you must provide a complete picture of your health. This allows for a truly personalized risk assessment.
Your Complete Medical History
Be prepared to discuss any personal or family history of specific conditions. This includes any allergies, pheochromocytoma (a type of tumor), seizures, heart disease, or issues with dizziness.
A Full List of Medications and Supplements
Selegiline, as a monoamine oxidase inhibitor (MAOI), can have dangerous interactions. Provide your doctor with a written list of every prescription medicine, over-the-counter drug, vitamin, and herbal supplement you take. Pay special attention to diet pills, cold medicines, or any supplements containing tyramine.
Your Lifestyle and Environment
Be open about your use of alcohol, which is not recommended while using selegiline. Also, inform your doctor of any upcoming surgeries.
Understanding the Trade-offs and Potential Risks
An objective decision requires acknowledging the risks on both sides of the equation—the risk of taking the medication and the risk of not taking it.
The Impact of Untreated Depression
Untreated depression in a new mother can have significant negative consequences for both her own health and the baby's development and bonding. Effective treatment is not an elective, it is a medical necessity.
Side Effects Affecting Caregiving
The selegiline patch may cause drowsiness or dizziness. This is a critical safety consideration for any parent caring for a newborn. You must not drive or operate machinery until you know how the medication affects you.
The Unknown Risk to the Infant
Because selegiline's effects on a nursing infant are unstudied, the default medical stance is one of caution. The primary risk is the unknown potential for the drug to cause adverse effects in your child.
Proper and Safe Application of the Patch
If you and your doctor decide to proceed, using the patch correctly is essential for safety and effectiveness.
Preparing the Application Site
Always apply the patch to a clean, dry, and smooth area of skin. Wash the area with soap and warm water and dry it thoroughly before application.
Where to Apply
Recommended locations are the upper chest, back, upper thigh, or the outer surface of the upper arm.
The 24-Hour Cycle
The patch must be changed every 24 hours. When you apply a new patch, choose a different location to prevent skin irritation. Rotating sites is crucial.
Key Safety Precautions
Apply the patch immediately after removing it from the pouch and press it firmly for about 30 seconds. Avoid exposing the application site to direct heat sources like heating pads or saunas. Always wash your hands after handling the patch.
Making the Right Choice for Your Goal
Your path forward will depend on your personal health needs and your collaborative decisions with your healthcare team.
- If your primary focus is maximizing safety due to the lack of data: You will need to discuss alternative depression treatments with your doctor that have a more established safety profile during breastfeeding.
- If selegiline is the most effective treatment for your specific needs: Your conversation must focus on a detailed risk-benefit analysis, which may include a plan to closely monitor your infant for any unusual changes in behavior or health.
Your well-being is a cornerstone of your baby's health, and an informed partnership with your healthcare provider is the best way to protect you both.
Summary Table:
| Key Consideration | Details |
|---|---|
| Safety Data | No adequate studies on effects in breast milk or on nursing infants. |
| Primary Risk | Unknown potential for adverse effects on the infant. |
| Core Decision Factor | Risk vs. Benefit: Treating maternal depression vs. unknown infant risk. |
| Essential Step | Thorough discussion and risk assessment with your healthcare provider. |
| If Proceeding | Correct patch application, site rotation, and monitoring for side effects is crucial. |
Need a reliable, high-quality transdermal patch for your pharmaceutical brand?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we understand that medication safety and precise formulation are paramount. Our technical expertise supports custom R&D and development to meet the specific needs of healthcare and pharma distributors and brands.
Let us help you develop safe and effective transdermal solutions. Contact our experts today to discuss your requirements.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
People Also Ask
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief

















