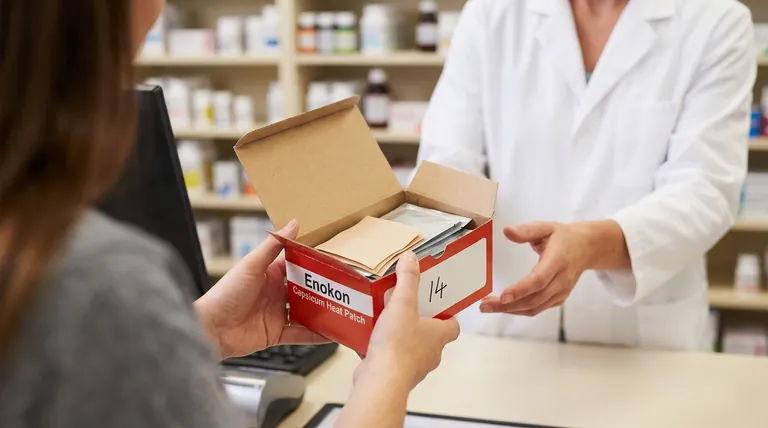
When returning fentanyl patches to a pharmacy, you must prepare them correctly to ensure they can be handled and disposed of safely. The process involves counting the number of used and unused patches, clearly indicating that number on the original container, and bringing the container directly to the dispensing pharmacy or an authorized medication return location.
The primary goal for returning fentanyl patches is to prevent accidental exposure, diversion, and environmental harm. A formal return process ensures these potent medications are tracked and destroyed safely by qualified professionals.
The Critical Importance of Proper Disposal
Handling the return of a controlled substance like fentanyl requires a level of diligence beyond that of other medications. The risks associated with improper disposal are significant, making the pharmacy return process essential.
Why You Cannot Throw Them Away
Fentanyl patches, even after use, contain a substantial amount of active medication.
Placing them in the household garbage creates a risk of accidental exposure to children, pets, or sanitation workers who may come into contact with them.
Flushing patches down the toilet is equally dangerous, as it introduces a powerful opioid into the water supply, harming the environment and potentially affecting public health.
Preventing Diversion and Misuse
Fentanyl is a potent and highly sought-after controlled substance.
Leaving unused or even used patches in the home poses a risk of theft and illegal diversion. Securely returning all patches to the pharmacy closes this loop, preventing the medication from falling into the wrong hands.
A Step-by-Step Guide for Safe Returns
Follow this simple procedure to ensure the safe and responsible return of your fentanyl patches. This protects you, the pharmacy staff, and the community.
Step 1: Prepare the Patches
For used patches, fold them in half so that the adhesive side sticks to itself. This minimizes the risk of accidental contact with any remaining medication.
Keep unused patches in their original, sealed pouches. Do not remove them.
Step 2: Use the Original Container
Place all patches—both used and unused—back into their original box, vial, or container provided by the pharmacy.
This keeps them securely together and properly identified.
Step 3: Count and Clearly Label
Count the exact number of patches you are returning.
Write this number clearly on the outside of the container. This simple step is crucial for the pharmacy's inventory and safe handling procedures.
Step 4: Return to an Authorized Location
Bring the prepared container directly to the pharmacy that dispensed the medication.
Alternatively, you can use an official medication take-back program or location if one is available in your area. Your pharmacist can provide information on local options.
Common Mistakes to Avoid
Proper handling doesn't end after the medication is used. Storage and disposal are key parts of the safety plan. Avoiding these common errors is critical.
Mistake 1: Improper Storage Before Return
Any unused patches must be stored securely to prevent accidental access or misuse.
Keep them in their sealed pouches at room temperature (below 25°C / 77°F), away from heat and light. Most importantly, they must be kept in a locked cabinet or safe, out of the reach of children and visitors.
Mistake 2: Disposing at Home
Never dispose of fentanyl patches in the garbage or toilet. The risks are too high.
The only safe and approved method of disposal is through a pharmacy or an authorized take-back program.
Making the Right Choice for Your Goal
Your specific situation will determine the exact steps you need to take.
- If you have USED patches to return: Fold each patch sticky-side-in, place them in the original container, and clearly label it with the total count before returning it to the pharmacy.
- If you have UNUSED patches to return: Keep them in their original sealed pouches, store them in a locked and secure location, and return them to the pharmacy as soon as possible.
- If you are unsure where to return medication: Always contact your dispensing pharmacy first, as they can provide the most accurate and safe guidance for your specific location.
By following these procedures, you take an active role in ensuring this powerful medication is managed safely from prescription to disposal.
Summary Table:
| Step | Action | Key Detail |
|---|---|---|
| 1 | Prepare Patches | Fold used patches sticky-side together. Keep unused patches sealed. |
| 2 | Use Original Container | Place all patches back into the original box or vial. |
| 3 | Count & Label | Write the total number of patches clearly on the container. |
| 4 | Return to Pharmacy | Take the container to the dispensing pharmacy or an authorized take-back location. |
Need a reliable and safe transdermal patch supplier? As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharma distributors, we ensure product safety and compliance from R&D to disposal. Benefit from our technical expertise for custom development. Contact our team today to discuss your needs.
Visual Guide
Related Products
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- What is the purpose of capsaicin patches? A Guide to Temporary Pain Relief
- Are natural and herbal pain relief patches effective and safe? Discover the Benefits of Targeted Relief
- Can the pain relief patch be used with other external analgesic products? A Critical Safety Guide
- What side effects might occur from using capsaicin patches? Understand the difference between normal sensation and danger signs.
- What precautions should be taken with buprenorphine patches? Ensure Safe Use and Avoid Overdose Risks













