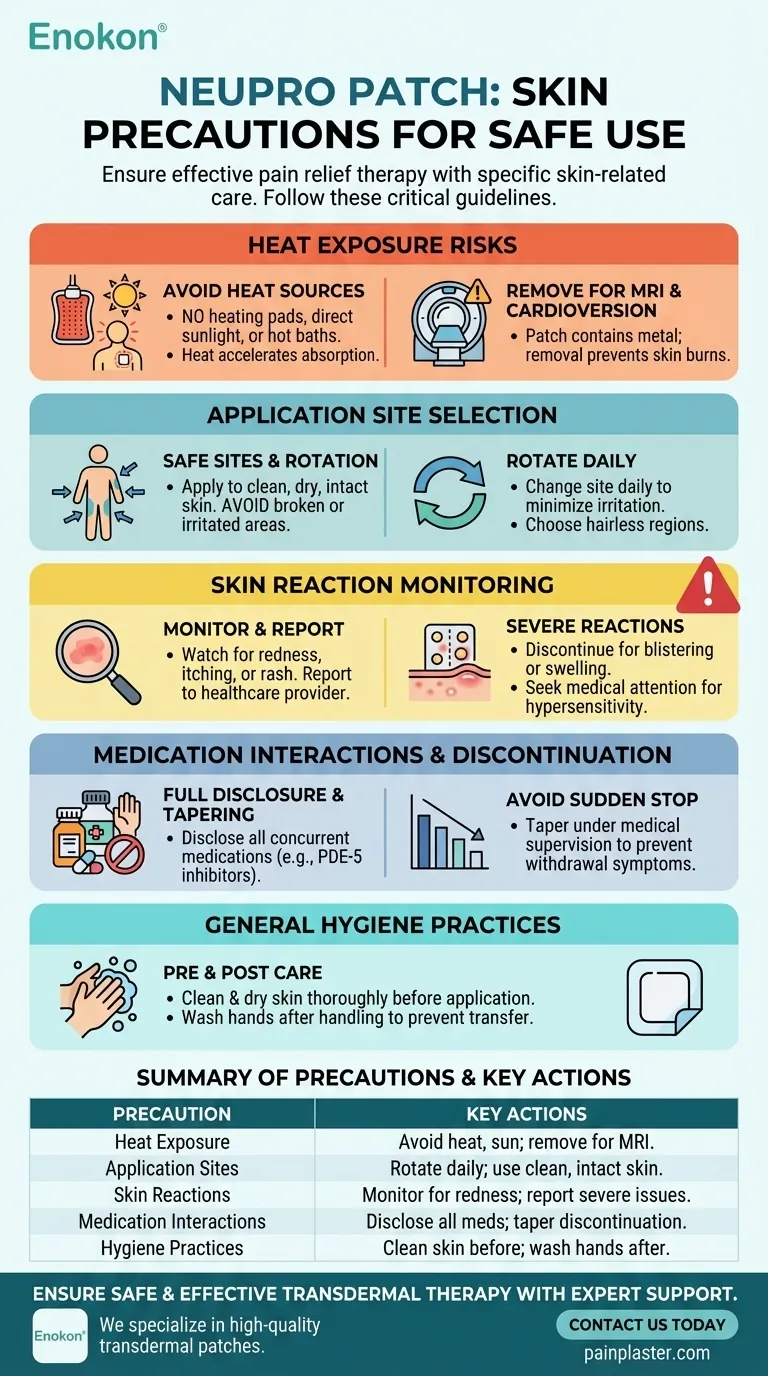
NEUPRO patches, a type of pain relief patches, require specific skin-related precautions to ensure safe and effective use. Key considerations include avoiding heat exposure, proper application techniques, and monitoring for skin reactions. Heat sources like heating pads or sunlight can increase medication absorption unpredictably, while MRI procedures or cardioversion may cause burns if patches are not removed. Skin irritation at application sites should be promptly reported, and patches should never be applied to broken or irritated skin. Additionally, patients must avoid sudden discontinuation and disclose all concurrent medications to healthcare providers to prevent adverse interactions.
Key Points Explained:
-
Heat Exposure Risks
- NEUPRO patches should not be exposed to external heat sources (e.g., heating pads, direct sunlight, hot baths). Heat accelerates drug absorption, potentially leading to overdose or inconsistent dosing.
- During MRI scans or cardioversion procedures, patches must be removed entirely to prevent skin burns caused by electrical currents or magnetic fields interacting with the patch's metallic components.
-
Application Site Selection
- Avoid applying patches to broken, cut, or irritated skin, as this may increase systemic absorption or cause localized reactions.
- Rotate application sites daily to minimize skin irritation. Preferred areas include the abdomen, thigh, hip, or upper arm—regions with minimal hair and friction.
-
Skin Reaction Monitoring
- Redness, itching, or rash at the application site should be documented and reported to a healthcare provider. Severe reactions (blistering, swelling) may require discontinuation.
- Hypersensitivity reactions, though rare, warrant immediate medical attention. A patch test may be recommended for patients with sensitive skin.
-
Medication Interactions & Discontinuation
- Sudden removal of NEUPRO patches can trigger withdrawal symptoms (e.g., agitation, muscle rigidity). Tapering under medical supervision is advised.
- Concurrent use with PDE-5 inhibitors (e.g., sildenafil) or other dopamine agonists may exacerbate side effects. Full medication history disclosure is critical.
-
General Hygiene Practices
- Clean and dry the skin thoroughly before application to ensure adhesion and reduce contamination risk.
- Wash hands after handling patches to prevent accidental transfer to eyes or mucous membranes.
These precautions highlight how small adjustments in daily routines—like checking weather forecasts for heat or setting reminders for patch rotation—can significantly impact treatment safety. By integrating these measures, patients harness the therapeutic benefits of NEUPRO while mitigating risks, embodying the delicate balance between innovation and vigilance in transdermal therapies.
Summary Table:
| Precaution | Key Actions |
|---|---|
| Heat Exposure | Avoid heating pads, sunlight, or hot baths; remove during MRI/cardioversion. |
| Application Sites | Rotate daily; use clean, intact skin (abdomen, thigh, hip, upper arm). |
| Skin Reactions | Monitor for redness/itching; report severe reactions (blistering/swelling). |
| Medication Interactions | Disclose all medications; avoid abrupt discontinuation. |
| Hygiene Practices | Clean/dry skin before application; wash hands after handling. |
Ensure Safe and Effective Transdermal Therapy with Expert Support
At Enokon, we specialize in manufacturing high-quality transdermal patches, including pain relief solutions like NEUPRO. Our technical expertise ensures reliable products tailored to your needs—whether you're a healthcare distributor or a brand seeking custom R&D.
Contact us today to discuss how we can enhance your product line with safe, innovative patch solutions!
Visual Guide
Related Products
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Heating Pain Relief Patches for Menstrual Cramps
- Prostate Pain Kidney Health Care Patch for Men
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
People Also Ask
- What are HRT patches? A Guide to Transdermal Hormone Therapy for Menopause Relief
- What are the benefits of HRT patches? Relieve Menopause Symptoms with Direct, Steady Relief
- What can be done if a skin reaction occurs with a specific HRT patch brand? Solutions & Alternatives
- What should be done if an HRT patch falls off? Quick Fixes & Prevention Tips
- What are potential side effects of HRT patches? A Guide to Managing Skin & Systemic Reactions
















