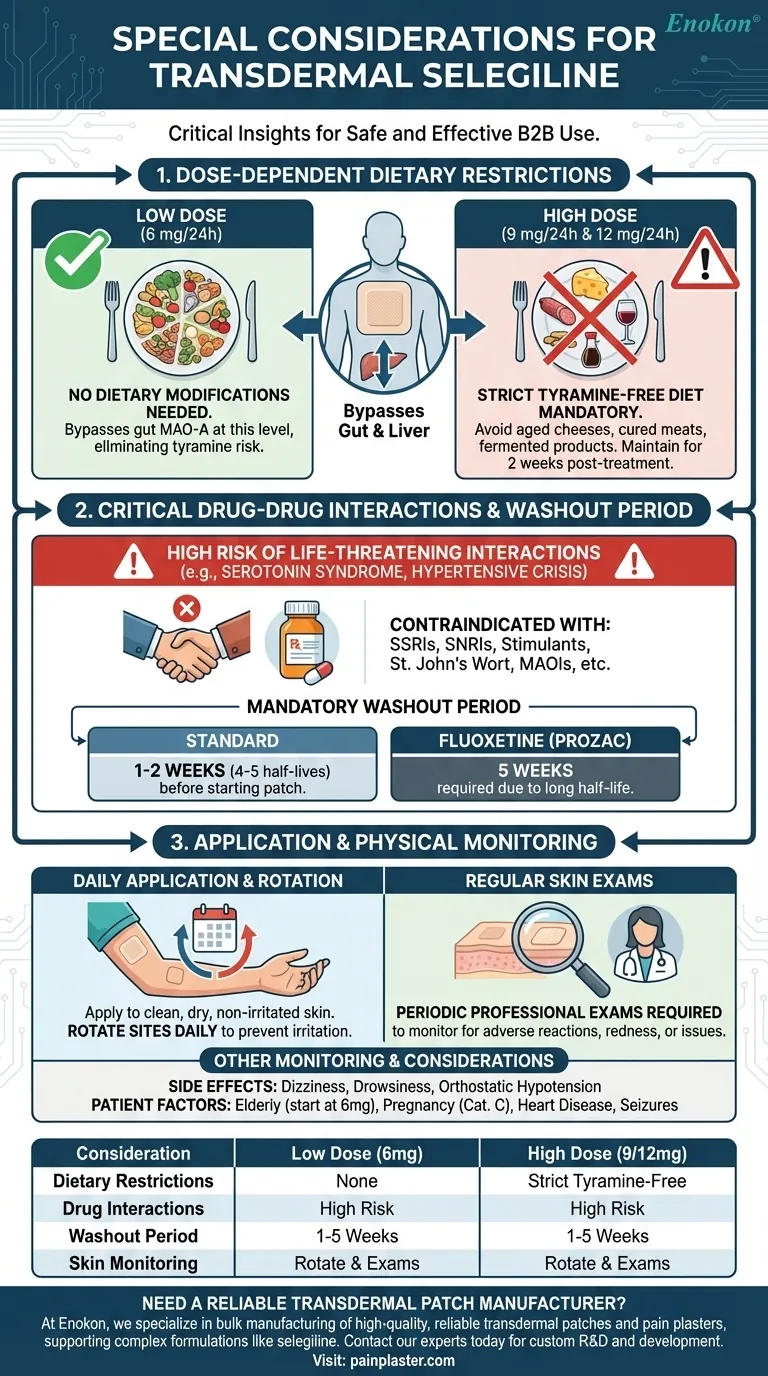
The most critical considerations for transdermal selegiline are its dose-dependent dietary restrictions, a high potential for dangerous drug-drug interactions requiring a "washout" period, and the need for regular skin examinations at the application site. Unlike its oral counterparts, the lowest dose of the selegiline patch (6 mg/24 hours) uniquely bypasses the gut's metabolic processes, eliminating the need to avoid tyramine-rich foods.
The selegiline patch offers a significant advantage at its lowest dose by avoiding the strict diet required by other MAOIs. However, this convenience does not reduce its risk of potentially fatal interactions with many common medications, making a thorough medication review the paramount safety consideration.
The Dose-Dependent Tyramine Restriction
The primary advantage of the transdermal system is how it alters the drug's interaction with monoamine oxidase (MAO) enzymes in the digestive system. This directly impacts the well-known dietary rules associated with this class of medication.
How the Patch Avoids the "Cheese Effect"
When taken orally, selegiline inhibits MAO-A enzymes in the gut. These enzymes are responsible for breaking down tyramine, an amino acid found in aged and fermented foods. Without this enzyme activity, tyramine levels can spike, causing a dangerous hypertensive crisis.
The transdermal patch delivers selegiline directly into the bloodstream, largely bypassing the gut and liver on its "first pass." This allows the drug to work in the brain without significantly impacting the MAO-A enzymes in the digestive tract, at least at lower doses.
The 6 mg/24 hours Dose
For patients using the starting dose of 6 mg/24 hours, no dietary modifications are necessary. This is the key clinical benefit that sets the transdermal patch apart from other MAOI antidepressants.
The 9 mg and 12 mg/24 hours Doses
Once the dose is increased to 9 mg or 12 mg per 24 hours, this benefit disappears. At these higher concentrations, enough selegiline circulates to inhibit MAO-A in the gut.
Patients on these doses must follow a strict tyramine-restricted diet. This includes avoiding foods like aged cheeses, cured meats (salami, pepperoni), fermented products (sauerkraut, soy sauce, tofu), and certain alcoholic beverages. This diet must be maintained throughout treatment and for two weeks after discontinuing the patch.
Critical Drug and Medical Interactions
The risk of drug interactions remains high regardless of the dose. These interactions can trigger life-threatening conditions like serotonin syndrome or hypertensive crisis.
High Risk of Serotonin Syndrome
Combining selegiline with other medications that increase serotonin is contraindicated. This includes most other antidepressants (like SSRIs and SNRIs), stimulants, and even some herbal supplements like St. John's Wort.
The Mandatory "Washout" Period
Because of these severe interaction risks, a "washout" period is required when switching to or from transdermal selegiline.
Discontinuing a contraindicated antidepressant requires a wait of 1-2 weeks (or 4-5 half-lives of the drug) before starting the selegiline patch. When stopping fluoxetine (Prozac), which has a very long half-life, this washout period is extended to five weeks.
Pre-existing Conditions and Other Factors
Patients must inform their doctor of their complete medical history. Selegiline may be contraindicated or require extreme caution in people with conditions like pheochromocytoma (a type of adrenal gland tumor), heart disease, or a history of seizures. It is also important to disclose any plans for surgery.
Application and Physical Monitoring
The delivery system itself introduces unique considerations that are not a factor with oral medications.
Proper Application and Site Monitoring
The patch should be applied once daily to a clean, dry, and non-irritated area of the skin. It is essential to rotate application sites to prevent skin irritation.
Required Skin Examinations
Beyond self-monitoring, patients require periodic skin examinations from their healthcare provider. This is to professionally assess the skin at application sites for any adverse reactions, which can range from mild redness to more significant dermatological issues.
Understanding the Trade-offs
Choosing transdermal selegiline involves balancing its unique benefits against its significant risks and complexities.
Side Effects to Monitor
Common side effects include dizziness, drowsiness, and orthostatic hypotension (a sudden drop in blood pressure causing fainting upon standing up). Caution is advised when driving or performing other tasks requiring alertness until you know how the medication affects you.
Special Population Considerations
Transdermal selegiline is classified as a Pregnancy Category C drug, meaning its risks to a fetus are not well-established and it should be used with caution. For patients aged 65 or older, the recommended dose is typically the lowest strength of 6 mg/24 hours.
How to Ensure Safe and Effective Use
Your approach to treatment will depend on your specific clinical situation.
- If you are starting treatment: The most crucial step is providing your doctor with a complete list of every medication, supplement, and herbal product you take to screen for dangerous interactions.
- If you are on the 6 mg dose: You can enjoy the freedom from dietary restrictions, but you must remain vigilant about avoiding all contraindicated medications and monitoring your skin.
- If your dose is increased to 9 mg or 12 mg: You must commit to the strict tyramine-free diet during treatment and for two weeks after stopping to prevent a hypertensive crisis.
- If you are switching from another antidepressant: Adhering precisely to the required multi-week washout period is non-negotiable for your safety.
Understanding these specific rules is the key to using this powerful medication safely and effectively.
Summary Table:
| Consideration | Low Dose (6 mg/24h) | High Dose (9 mg/24h, 12 mg/24h) |
|---|---|---|
| Dietary Restrictions | None required | Strict tyramine-free diet mandatory |
| Drug Interactions | High risk (SSRIs, SNRIs, stimulants) | High risk (SSRIs, SNRIs, stimulants) |
| Washout Period | 1-5 weeks when switching antidepressants | 1-5 weeks when switching antidepressants |
| Skin Monitoring | Rotate sites; regular professional exams | Rotate sites; regular professional exams |
Need a reliable transdermal patch manufacturer for your healthcare or pharma brand?
At Enokon, we specialize in the bulk manufacturing of high-quality, reliable transdermal patches and pain plasters. Our technical expertise supports custom R&D and development to meet your specific formulation needs, including complex delivery systems like those used for medications such as selegiline.
Contact our experts today to discuss how we can partner to bring safe and effective transdermal solutions to your market.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief















