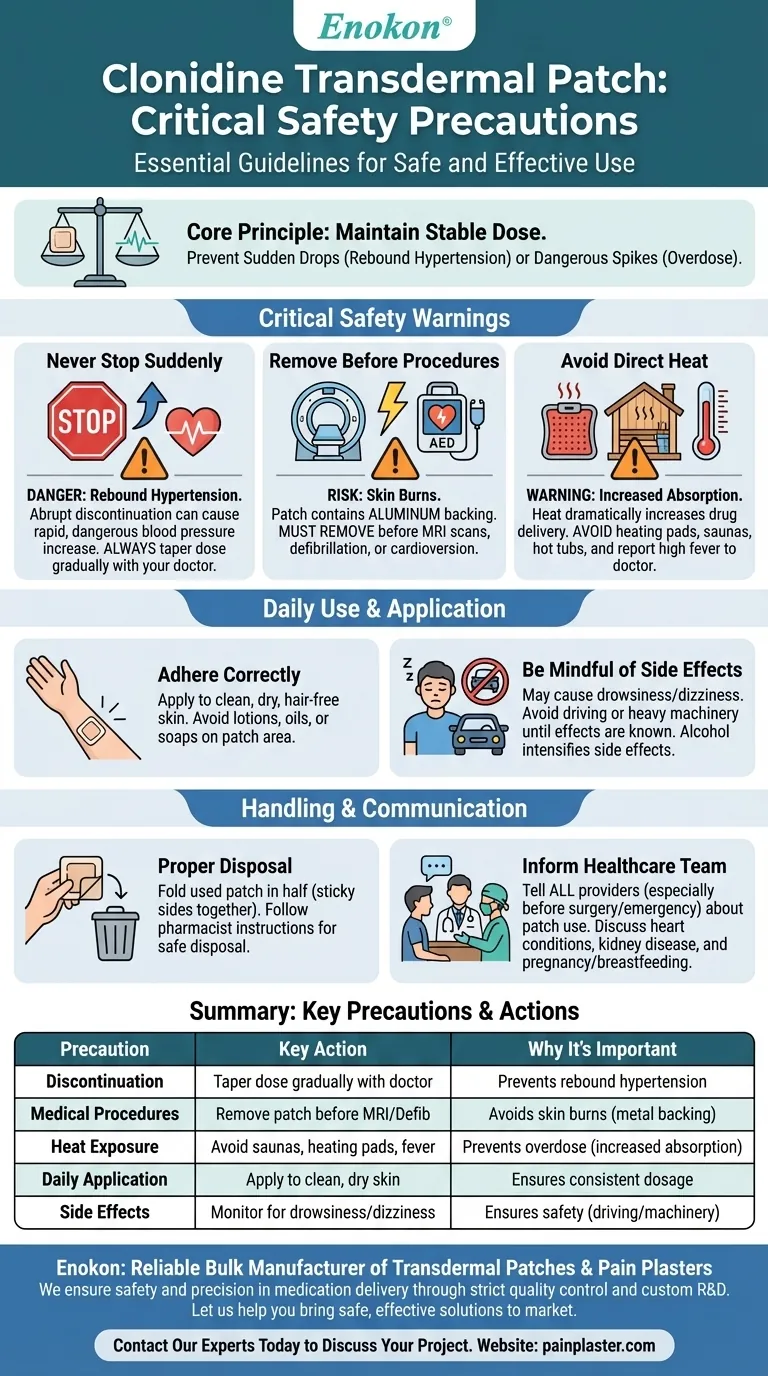
Using a clonidine transdermal patch requires careful attention to specific precautions that go beyond typical medication use. The most critical warnings involve never stopping the medication abruptly, removing the patch before certain medical procedures like MRIs to prevent burns, and avoiding heat sources which can dangerously increase drug absorption.
The core principle behind clonidine patch safety is maintaining a stable, controlled dose. Many precautions are designed to prevent either a sudden drop in medication levels (from stopping suddenly) or a dangerous spike (from heat exposure or procedural interactions).
Critical Safety Warnings You Must Understand
These precautions are essential to prevent serious adverse events. Understanding the "why" behind each warning is key to using this medication safely.
Never Stop Suddenly
Abruptly discontinuing clonidine can cause a rapid, dangerous increase in blood pressure known as rebound hypertension.
Your body becomes accustomed to the medication. Stopping suddenly can lead to symptoms like nervousness, headache, and tremor. Always consult your doctor to taper off the dose gradually.
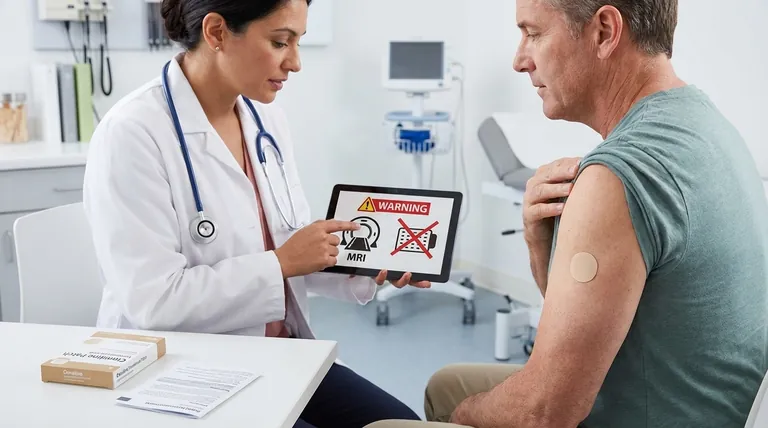
Remove Before Certain Medical Procedures
The clonidine patch contains an aluminum backing. This layer can conduct electricity and heat, posing a significant risk during specific medical events.
You must remove the patch before an MRI scan to avoid skin burns at the patch site. It must also be removed before defibrillation or cardioversion for the same reason.
Avoid Direct Heat and Fever
Heat dramatically increases the absorption of clonidine through your skin, which can lead to an overdose.
Avoid placing heating pads over the patch. Stay away from extended time in hot tubs, saunas, or very hot baths. Be aware that a high fever can also increase absorption, so contact your doctor if you become ill.
Daily Use and Application Precautions
Proper daily habits ensure the patch works effectively and safely.
Adhering to Your Skin Correctly
The area of skin where you apply the patch should be clean, dry, and free of hair.
Do not use lotions, oils, soaps, or other products on the patch area, as they can prevent it from sticking properly and delivering a consistent dose.
Be Mindful of Side Effects
Clonidine can cause drowsiness and dizziness, which may impair your thinking and reaction times.
Avoid driving or operating heavy machinery until you know how the medication affects you. Be cautious when consuming alcohol, as it can intensify these side effects.
Proper Handling and Disposal
Always wash your hands after applying or removing a patch to avoid transferring medication.
When you remove a used patch, fold it in half with the sticky sides together. This seals in any remaining medication, preventing accidental exposure to children or pets. Follow your pharmacist's instructions for proper disposal.
Communicating with Your Healthcare Team
Your clonidine use impacts other aspects of your health and treatment. Open communication is vital.
Inform All Healthcare Providers
It is crucial that any doctor, dentist, or surgeon who treats you knows you are using a clonidine patch. This is especially important if you are scheduled for surgery or an emergency procedure.
Discuss Pre-existing Conditions
Clonidine can affect certain medical conditions. Be sure your doctor knows if you have heart problems like AV block, kidney disease, or a history of allergic reactions to adhesives.
Pregnancy and Breastfeeding
The effects of clonidine on an unborn baby are not fully known, and the drug can pass into breast milk.
Inform your doctor immediately if you are pregnant, plan to become pregnant, or are breastfeeding.
How to Apply This to Your Goal
Your approach to these precautions depends on your immediate focus.
- If your primary focus is day-to-day safety: Be most vigilant about avoiding heat, monitoring for drowsiness, and applying the patch to clean, dry skin.
- If your primary focus is preparing for a medical event: Your top priority is to inform all providers and remember to remove the patch before any MRI, defibrillation, or cardioversion.
- If your primary focus is treatment consistency: Never let your prescription run out to avoid an abrupt stop, and follow a gradual taper schedule with your doctor if you need to discontinue.
By understanding these principles, you can manage your treatment safely and effectively.
Summary Table:
| Precaution | Key Action | Why It's Important |
|---|---|---|
| Discontinuation | Taper dose gradually with a doctor | Prevents dangerous rebound hypertension |
| Medical Procedures | Remove patch before MRI/defibrillation | Avoids skin burns from metal backing |
| Heat Exposure | Avoid saunas, heating pads, high fever | Prevents overdose from increased absorption |
| Daily Application | Apply to clean, dry, hair-free skin | Ensures consistent, correct dosage |
| Side Effects | Monitor for drowsiness/dizziness | Ensures safety for driving/operating machinery |
Need a reliable manufacturer for your transdermal patch products?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we understand the critical importance of safety and precision in medication delivery. Our technical expertise ensures every patch is developed with strict quality control for consistent dosing and patient safety.
We specialize in custom R&D and development for healthcare and pharma distributors and brands. Let us help you bring safe, effective transdermal solutions to market.
Contact our experts today to discuss your project requirements and benefit from our manufacturing expertise.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use














