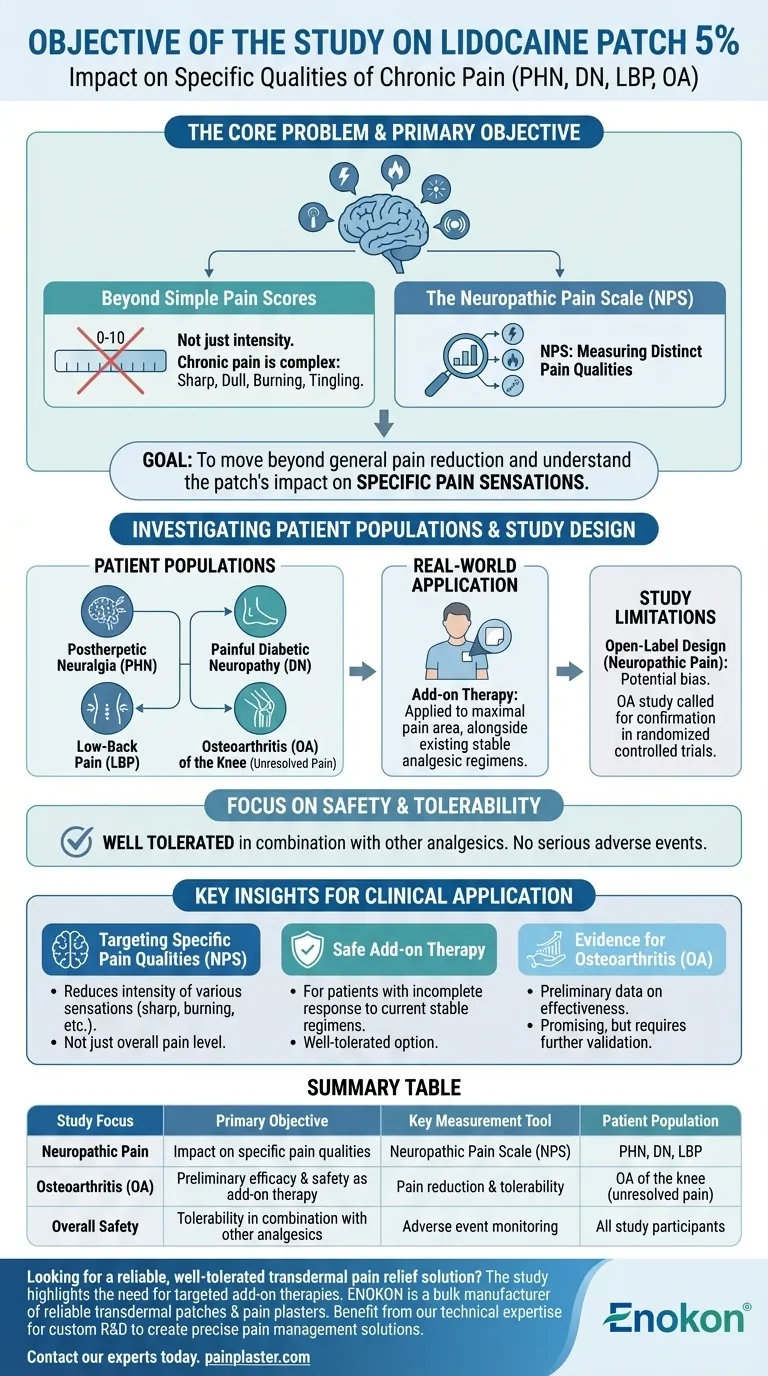
The primary objective of the study was to determine the impact of the lidocaine patch 5% on the specific qualities of chronic pain associated with postherpetic neuralgia (PHN), painful diabetic neuropathy (DN), and low-back pain (LBP). Researchers used the Neuropathic Pain Scale (NPS) to measure these changes. A related objective in separate studies was to assess the patch's effectiveness for patients with osteoarthritis.
The core goal of this research was not simply to confirm pain reduction, but to understand the patch's specific impact on the qualities of chronic pain and to evaluate it as a well-tolerated add-on therapy for patients with unresolved pain.
The Core Problem: Measuring a Complex Sensation
Beyond Simple Pain Scores
Chronic pain is not a single sensation. It can be sharp, dull, burning, or tingling, and its intensity can fluctuate.
The study’s objective was to move beyond a simple "0-to-10" pain rating and measure the patch's effect on these distinct pain qualities.
The Neuropathic Pain Scale (NPS)
To achieve this, researchers used the Neuropathic Pain Scale (NPS). This specialized tool is designed to capture the different dimensions of nerve-related pain.
Using the NPS allowed the study to provide a more detailed picture of how the lidocaine patch was changing the patient's experience of pain.
Targeting Unresolved Pain
A key aspect of the research objective, particularly in the osteoarthritis studies, was to evaluate patients who had an incomplete response to their existing, stable analgesic regimens.
This shows the goal was to see if the lidocaine patch could fill a therapeutic gap for those not getting adequate relief from their current treatments.
Investigating Specific Patient Populations
Neuropathic Pain Conditions
One primary study focused on three common and difficult-to-treat chronic pain conditions:
- Postherpetic neuralgia (PHN): Lingering nerve pain after a shingles infection.
- Painful diabetic neuropathy (DN): Nerve damage caused by diabetes.
- Low-back pain (LBP): A common condition with potential neuropathic components.
Osteoarthritis of the Knee
Other studies specifically aimed to assess effectiveness in patients with osteoarthritis of the knee.
The stated goal here was to see if the patch provided enough benefit to warrant larger, more rigorous randomized controlled trials in the future.
Real-World Application
The study design mimicked typical clinical use. Patients applied up to four patches daily to the area of maximal pain while continuing their other pain medications without changes.
This approach was intended to determine the patch's effectiveness and safety as part of a combined, multi-modal treatment plan.
Understanding the Study's Limitations
Open-Label Design
The primary study on neuropathic pain was open-label and non-randomized. This means both researchers and patients knew who was receiving the lidocaine patch.
While useful for gathering initial data, this design can introduce bias and is generally considered less rigorous than a double-blind, placebo-controlled trial.
A Call for Confirmation
Researchers involved in the osteoarthritis study explicitly stated that their objective was to determine if more definitive trials were warranted.
This acknowledges that their initial findings, while positive, required confirmation from more robust clinical trials to establish a higher level of evidence.
Focus on Safety and Tolerability
A critical secondary objective was to evaluate the patch's safety profile. The study found it was well tolerated in combination with other analgesics.
No serious adverse events or negative drug interactions were reported, supporting its use as an add-on therapy.
Making the Right Choice for Your Goal
Based on the study's specific objectives, we can draw several key insights for clinical application.
- If your primary focus is treating specific neuropathic pain qualities: The research was designed to show the lidocaine patch 5% can reduce the intensity of various pain sensations, not just the overall pain level.
- If your primary focus is finding a safe add-on therapy: The study aimed to prove the patch is a well-tolerated option for patients already on a stable analgesic regimen who still experience persistent pain.
- If your primary focus is evidence for osteoarthritis: The research objective was to gather preliminary data on effectiveness, positioning the patch as a promising option that requires further validation through controlled trials.
Ultimately, the collective objective of these studies was to establish the lidocaine patch 5% as a targeted and safe tool for managing localized, chronic pain that is not fully controlled by other treatments.
Summary Table:
| Study Focus | Primary Objective | Key Measurement Tool | Patient Population |
|---|---|---|---|
| Neuropathic Pain | Impact on specific pain qualities (sharp, burning, etc.) | Neuropathic Pain Scale (NPS) | PHN, Diabetic Neuropathy, Low-Back Pain |
| Osteoarthritis (OA) | Preliminary efficacy & safety as add-on therapy | Pain reduction & tolerability | OA of the knee with unresolved pain |
| Overall Safety | Tolerability in combination with other analgesics | Adverse event monitoring | All study participants |
Looking for a reliable, well-tolerated transdermal pain relief solution?
The study on lidocaine patch 5% highlights the need for targeted, safe add-on therapies for complex chronic pain. At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharma distributors and brands. Benefit from our technical expertise for custom R&D and development to create the precise pain management solutions your patients need.
Contact our experts today to discuss your specific requirements and how we can support your product development.
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Menthol Gel Pain Relief Patch
People Also Ask
- Are lidocaine patches safe to use during pregnancy? A Guide to Making an Informed Choice
- How can you use lidocaine patches for multiple sore spots? A Guide to Safe, Effective Pain Relief
- How does the lidocaine patch work? Targeted Relief for Nerve Pain Explained
- For what condition are lidocaine patches approved in the United Kingdom? A Guide to Postherpetic Neuralgia Treatment
- What systemic side effects can lidocaine patches cause? Minimizing Risks for Safe Pain Relief















