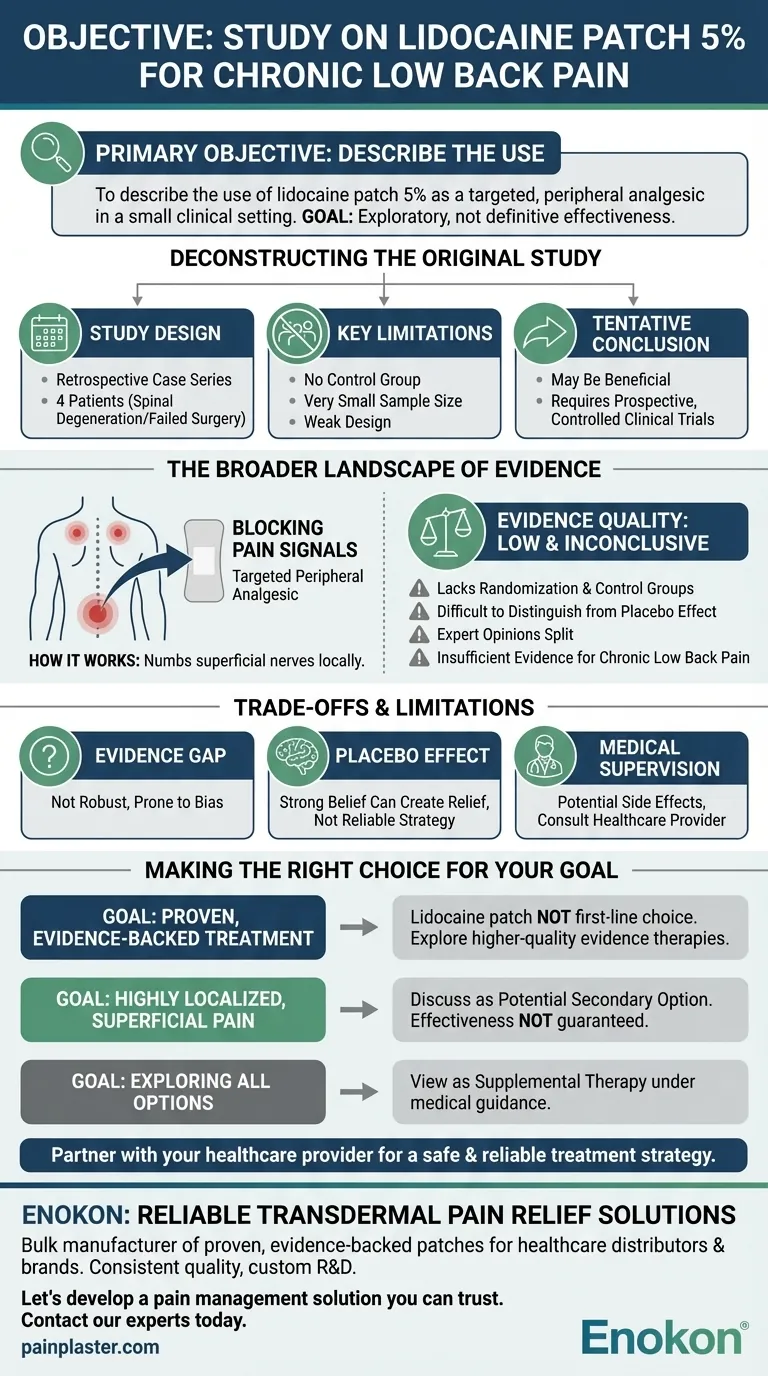
The primary objective of the study was to describe the use of the lidocaine patch 5% as a targeted, peripheral analgesic in treating patients with chronic low back pain. The goal was not to definitively prove its effectiveness but rather to report on its application in a small clinical setting.
While the initial study aimed to describe how the lidocaine patch was used, the crucial takeaway from the broader body of research is that its effectiveness for chronic low back pain remains unproven. The existing evidence is considered low-quality and inconclusive, making it a questionable primary treatment option.

Deconstructing the Original Study
The study in question served as an initial observation, not a definitive clinical trial. Understanding its design is key to interpreting its findings correctly.
The Stated Goal
The objective was simply to describe the use of the patch. This is an exploratory goal common in early research, designed to generate hypotheses for future, more rigorous testing.
A Look at the Methods
This research was a retrospective case series. It looked back at the medical records of just four patients who were treated for pain from spinal degeneration and complications of failed back surgery.
This type of study design is considered weak because it has no control group to compare results against and involves a very small number of participants.
The Tentative Conclusion
The study concluded that adding the lidocaine patch to a pain management plan may be beneficial. However, the authors themselves noted that prospective, controlled clinical trials were necessary to properly evaluate its efficacy and safety.
The Broader Landscape of Evidence
When we move beyond that initial case series, the overall scientific consensus on lidocaine patches for low back pain is cautious and divided.
How Lidocaine Patches Work
The patch delivers a local anesthetic, lidocaine, through the skin. Its purpose is to numb the superficial nerves in a specific area, thereby blocking pain signals before they reach the brain. It is considered a targeted peripheral analgesic because it acts locally rather than systemically.
The Problem with Existing Research
Much of the research supporting lidocaine patches for back pain lacks randomization and control groups. Without these elements, it is difficult to distinguish the true effect of the medication from the powerful placebo effect.
Consequently, expert opinions are split. Some guidelines state there is insufficient evidence to either support or oppose their use for chronic low back pain.
Who Might Benefit?
Some evidence suggests the patches may offer relief for individuals with very localized pain or those with a condition of increased pain sensitivity. However, these potential benefits are not yet supported by high-quality studies.
Understanding the Trade-offs and Limitations
Trusting a treatment requires a clear-eyed view of its downsides and the quality of evidence supporting it. This is especially true for chronic pain management.
The Quality of Evidence is Low
The fundamental issue is that the evidence is simply not robust. Small, uncontrolled studies are prone to bias and are not sufficient to establish a treatment as a standard of care.
The Influence of the Placebo Effect
The physical sensation of applying a patch can create a strong belief that the treatment is working. While placebo-related pain relief is real, it's not a reliable or lasting therapeutic strategy and complicates the results of studies that don't use a placebo control group.
The Need for Medical Supervision
While generally safe, lidocaine patches can cause side effects ranging from mild skin irritation to, in rare cases, more severe systemic reactions. It is critical to work with a doctor to determine the underlying cause of your pain and the most appropriate treatment plan.
Making the Right Choice for Your Goal
Based on the current evidence, here is how to approach the use of a lidocaine patch for chronic low back pain.
- If your primary focus is a proven, evidence-backed treatment: The lidocaine patch is not a first-line choice, as higher-quality evidence supports other therapies.
- If you have highly localized, superficial pain: You might discuss the patch with your doctor as a potential secondary option, understanding that its effectiveness is not guaranteed.
- If you are exploring all options for pain management: View the lidocaine patch as a supplemental therapy to be tried under medical guidance, not as a core solution for chronic low back pain.
Ultimately, partnering with your healthcare provider is the most effective path to identifying a safe and reliable treatment strategy for your specific condition.
Summary Table:
| Aspect | Detail |
|---|---|
| Study Type | Retrospective Case Series |
| Primary Objective | To describe the use of the patch in a small clinical setting |
| Patient Population | 4 patients with pain from spinal degeneration/failed back surgery |
| Key Limitation | No control group; very small sample size |
| Overall Evidence Quality | Low-quality and inconclusive for chronic low back pain |
Need a reliable, high-quality transdermal pain relief solution?
As a bulk manufacturer of proven transdermal patches and pain plasters, Enokon provides healthcare and pharma distributors and brands with the reliable, evidence-backed products their customers need. Our technical expertise ensures consistent quality and supports custom R&D for your specific requirements.
Let's develop a pain management solution you can trust. Contact our experts today to discuss your needs.
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Menthol Gel Pain Relief Patch
People Also Ask
- How are lidocaine patches typically used for pain relief during pregnancy? A Guide to Safe, Targeted Relief
- How does the lidocaine patch work? Targeted Relief for Nerve Pain Explained
- Are lidocaine patches safe to use during pregnancy? A Guide to Making an Informed Choice
- Is it safe to use lidocaine patches while breastfeeding? Expert Guidance for Nursing Mothers
- When should someone contact a doctor regarding lidocaine patch use? Ensure Safe Pain Relief















