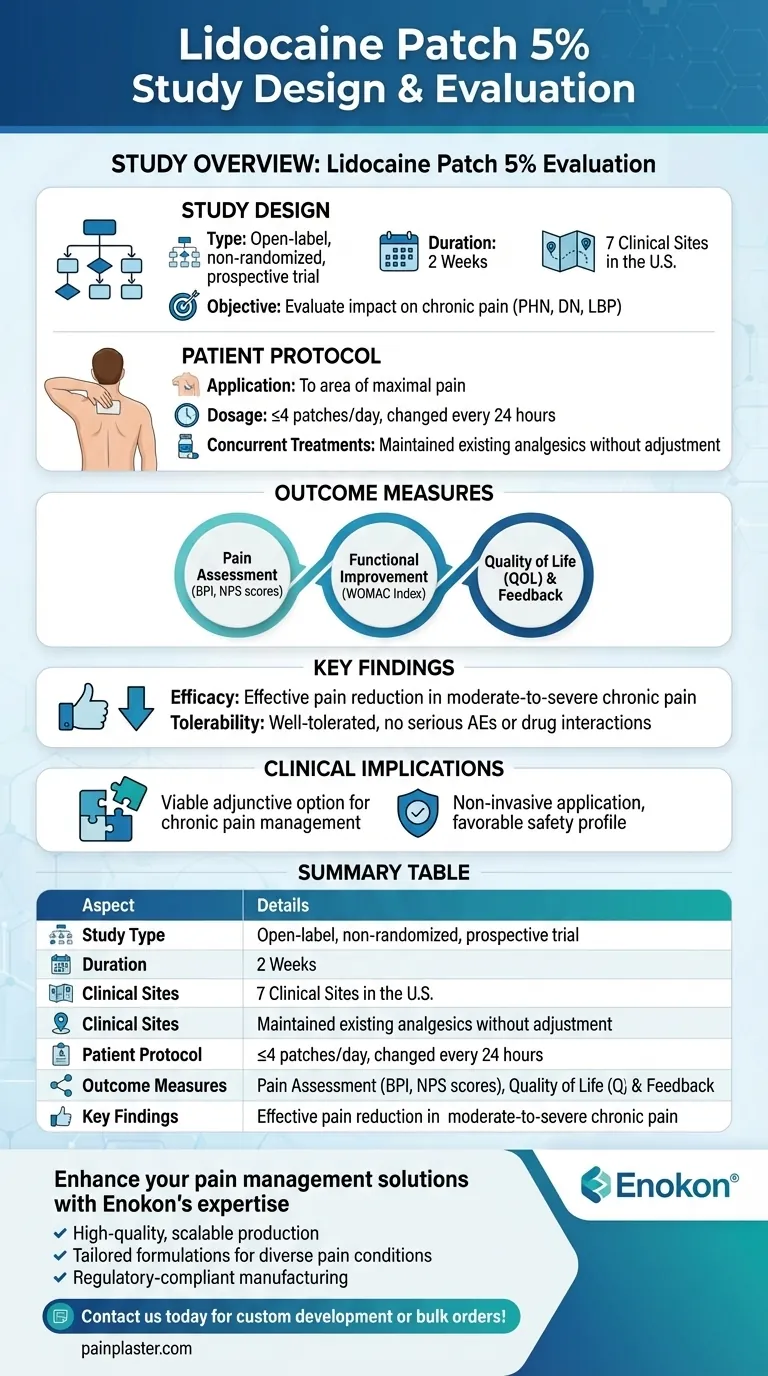
The study evaluating the lidocaine patch 5 percent was designed as an open-label, non-randomized, prospective trial conducted over two weeks across seven clinical sites in the U.S. Patients with chronic pain conditions applied the patch to areas of maximal pain, adhering to a regimen of no more than four patches changed every 24 hours, while continuing their existing analgesic treatments. Outcome measures included pain intensity, functional improvement, and quality of life assessments, demonstrating the patch's efficacy and tolerability in managing moderate-to-severe chronic pain.

Key Points Explained:
-
Study Design:
- Type: Open-label, non-randomized, prospective trial.
- Duration: 2 weeks.
- Sites: Conducted across 7 clinical trial sites in the United States.
- Objective: To evaluate the impact of the lidocaine patch 5% on chronic pain conditions, including postherpetic neuralgia (PHN), painful diabetic neuropathy (DN), and low-back pain (LBP).
-
Patient Protocol:
- Application: Patients applied the patch to the area of maximal pain.
- Dosage: No more than 4 patches per day, changed every 24 hours.
- Concurrent Treatments: Patients maintained their existing analgesic regimens without dose adjustments, allowing for assessment of the patch's additive effects.
-
Outcome Measures:
-
Pain Assessment:
- Brief Pain Inventory (BPI) scores for pain control.
- Neuropathic Pain Scale (NPS) for evaluating pain qualities.
- Functional Improvement: Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index.
- Patient/Investigator Feedback: Global assessment of patch satisfaction.
- Quality of Life (QOL): Assessments to gauge overall well-being and daily functioning.
-
Pain Assessment:
-
Findings:
- Efficacy: The patch effectively reduced pain intensity in patients with moderate-to-severe chronic pain from PHN, DN, or LBP.
- Tolerability: Well-tolerated in combination with other analgesics, with no serious adverse events (AEs) or drug interactions reported.
-
Clinical Implications:
- The study supports the lidocaine patch 5% as a viable option for managing chronic pain, particularly in patients who require adjunctive therapy without disrupting their current treatment plans. Its non-invasive application and favorable safety profile make it a practical choice for long-term pain management. Have you considered how such patches could integrate into broader pain management protocols?
Summary Table:
| Aspect | Details |
|---|---|
| Study Type | Open-label, non-randomized, prospective trial |
| Duration | 2 weeks |
| Clinical Sites | 7 sites in the U.S. |
| Patient Protocol | ≤4 patches/day, applied to maximal pain area; concurrent analgesics allowed |
| Outcome Measures | Pain intensity (BPI, NPS), functional improvement (WOMAC), QOL assessments |
| Key Findings | Effective pain reduction; well-tolerated with no serious AEs |
Enhance your pain management solutions with Enokon’s expertise
As a trusted bulk manufacturer of transdermal patches and pain plasters, Enokon partners with healthcare distributors and pharma brands to deliver reliable, custom-formulated solutions. Our technical R&D team ensures optimal efficacy and safety for chronic pain therapies like lidocaine patches.
Why choose Enokon?
✔ High-quality, scalable production
✔ Tailored formulations for diverse pain conditions
✔ Regulatory-compliant manufacturing
Contact us today to discuss custom development or bulk orders!
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Menthol Gel Pain Relief Patch
People Also Ask
- When should someone contact a doctor regarding lidocaine patch use? Ensure Safe Pain Relief
- How are lidocaine patches typically used for pain relief during pregnancy? A Guide to Safe, Targeted Relief
- How can you use lidocaine patches for multiple sore spots? A Guide to Safe, Effective Pain Relief
- How should the treated area be protected while wearing a lidocaine patch? Safety Tips for Effective Pain Relief
- How does the lidocaine patch work? Targeted Relief for Nerve Pain Explained















