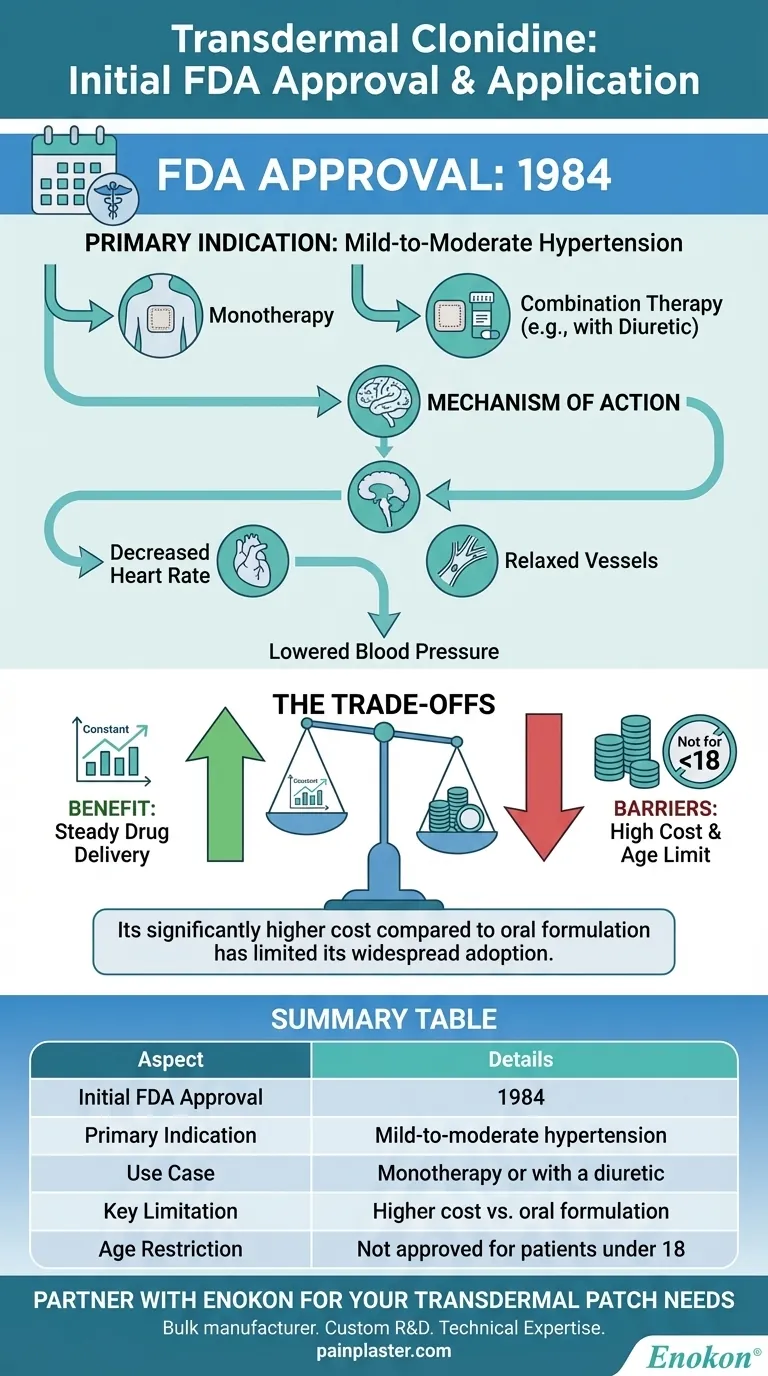
To be clear, transdermal clonidine was first approved by the US Food and Drug Administration (FDA) in 1984. Its specific, initial indication was for the treatment of mild-to-moderate hypertension, used either as a standalone therapy or in combination with a diuretic.
While its formal approval is for hypertension, the true value of the transdermal patch lies in its steady drug delivery. However, its significantly higher cost compared to the oral formulation has historically limited its widespread adoption for this primary purpose.
The Original Indication: A Focus on Hypertension
The approval of transdermal clonidine marked an important step in offering an alternative delivery system for a well-established medication. The goal was to provide consistent blood pressure control over several days with a single application.
FDA Approval in 1984
The system was officially indicated for managing hypertension. This approval covered patients with mild to moderate levels of high blood pressure.
Use as Monotherapy or Combination
The initial approval specified that transdermal clonidine could be used effectively on its own. It also noted its utility when combined with other antihypertensive agents, particularly diuretics.
How Transdermal Clonidine Works
Understanding its mechanism is key to understanding its purpose. It targets the central nervous system to achieve its effects on blood pressure.
The Alpha-Agonist Mechanism
Clonidine belongs to a class of drugs known as centrally acting alpha-agonist hypotensive agents. Its primary action is within the brain.
Impact on the Cardiovascular System
By stimulating specific receptors in the brainstem, clonidine reduces sympathetic outflow from the central nervous system. This action decreases heart rate and relaxes blood vessels, allowing blood to flow more easily and thereby lowering blood pressure.
Understanding the Trade-offs
Despite its therapeutic advantages, the transdermal formulation is not the default choice for every patient. Practical considerations play a major role in its clinical use.
The Cost Factor
The most significant barrier to broader use is its considerably higher cost when compared to the oral tablet form of clonidine. This economic factor often outweighs the benefit of a steady-state delivery system for many patients.
Approval Limitations
It is also important to note that transdermal clonidine is not approved for use by anyone younger than 18 years old.
Key Considerations for Clinical Context
To apply this knowledge, it's helpful to frame it based on your specific goal.
- If your primary focus is foundational history: Remember that transdermal clonidine's original and specific FDA approval in 1984 was for treating mild-to-moderate hypertension.
- If your primary focus is practical application: Recognize that while effective, its high cost relative to oral clonidine is the primary reason it is not a first-line therapy for most hypertension cases.
Understanding its original purpose and practical limitations provides a clear picture of transdermal clonidine's place in modern therapy.
Summary Table:
| Aspect | Details |
|---|---|
| Initial FDA Approval | 1984 |
| Primary Indication | Mild-to-moderate hypertension |
| Use Case | Monotherapy or with a diuretic |
| Key Limitation | Higher cost vs. oral formulation |
| Age Restriction | Not approved for patients under 18 |
Partner with Enokon for Your Transdermal Patch Needs
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon offers healthcare and pharma distributors and brands the technical expertise for custom R&D and development. Benefit from our proven capabilities in creating effective drug delivery systems.
Contact us today to discuss how we can support your transdermal product development.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Herbal Eye Protection Patch Eye Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
















