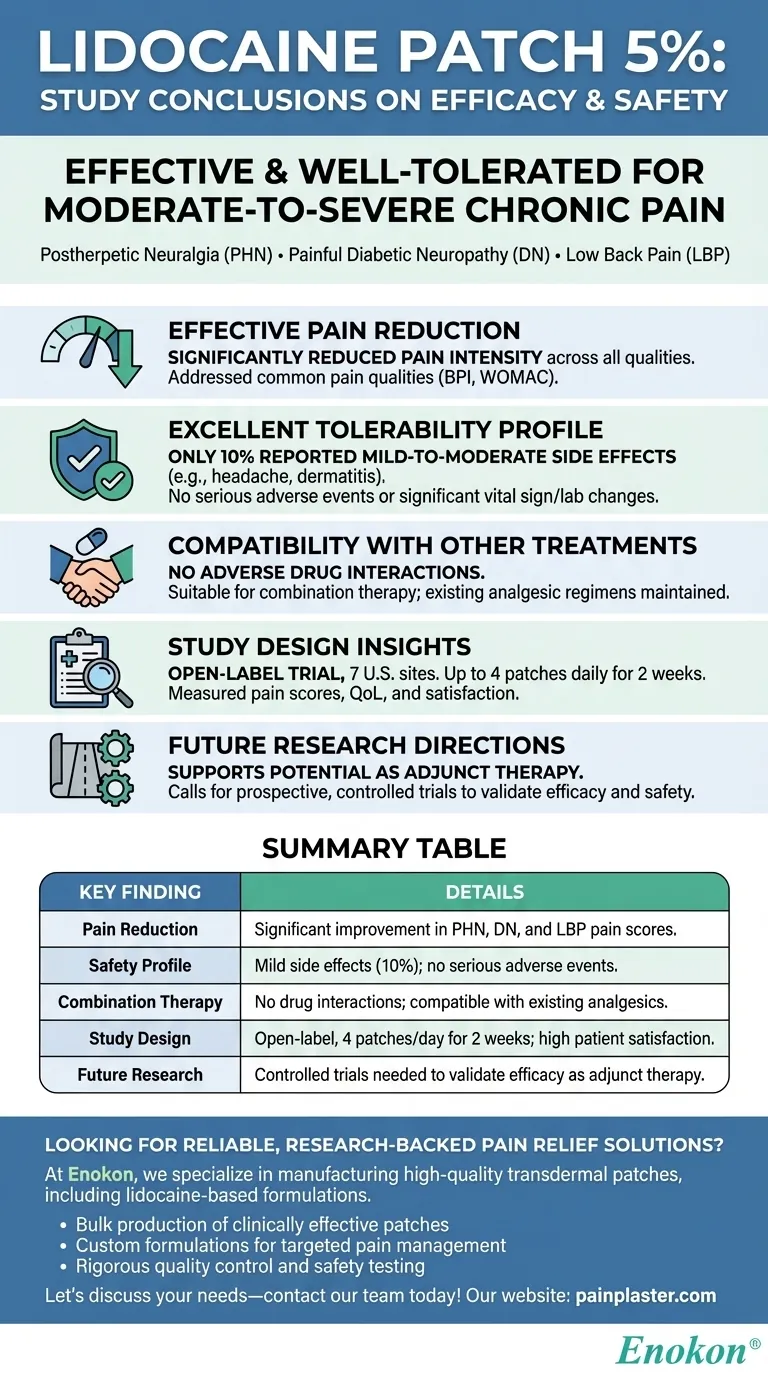
The study on the lidocaine patch 5 percent concluded that it is an effective and well-tolerated treatment for moderate-to-severe chronic pain conditions like postherpetic neuralgia (PHN), painful diabetic neuropathy (DN), and low back pain (LBP). It significantly reduced pain intensity across all measured qualities without causing serious adverse effects or drug interactions. The patch demonstrated good tolerability, with only mild-to-moderate side effects reported in a small percentage of patients. Researchers noted its potential benefits when added to existing analgesic regimens, though they recommended further controlled trials to confirm these findings.
Key Points Explained:
-
Effective Pain Reduction
- The lidocaine patch 5% significantly reduced pain intensity in patients with PHN, DN, or LBP.
- It addressed all common pain qualities measured by standardized tools like the Brief Pain Inventory (BPI) and WOMAC Osteoarthritis Index.
-
Excellent Tolerability Profile
- Only 10% of patients reported mild-to-moderate side effects (e.g., headache, dermatitis).
- No serious adverse events or clinically significant changes in vital signs/lab values occurred.
- The vehicle patch (placebo) also showed low adverse event rates, confirming the formulation's safety.
-
Compatibility with Other Treatments
- Patients maintained their existing analgesic regimens without dose adjustments.
- No adverse drug interactions were observed, making it suitable for combination therapy.
-
Study Design Insights
- Conducted as an open-label, non-randomized trial across 7 U.S. sites.
- Patients applied up to 4 patches daily to the most painful areas for two weeks.
- Outcome measures included pain scores, quality of life assessments, and patient/investigator satisfaction ratings.
-
Future Research Directions
- Researchers emphasized the need for prospective, controlled trials to validate efficacy and safety further.
- The preliminary data supports its potential as an adjunct therapy for chronic pain management.
Have you considered how localized delivery via patches could minimize systemic side effects compared to oral pain medications? This study highlights a promising shift toward targeted, non-invasive pain relief options.
Summary Table:
| Key Finding | Details |
|---|---|
| Pain Reduction | Significant improvement in PHN, DN, and LBP pain scores. |
| Safety Profile | Mild side effects (10% of patients); no serious adverse events. |
| Combination Therapy | No drug interactions; compatible with existing analgesics. |
| Study Design | Open-label trial with 4 patches/day for 2 weeks; high patient satisfaction. |
| Future Research | Controlled trials needed to validate efficacy as adjunct therapy. |
Looking for reliable, research-backed pain relief solutions?
At Enokon, we specialize in manufacturing high-quality transdermal patches, including lidocaine-based formulations, tailored for healthcare distributors and brands. Our expertise in custom R&D ensures optimal drug delivery, patient comfort, and regulatory compliance.
✅ Why partner with us?
- Bulk production of clinically effective patches
- Custom formulations for targeted pain management
- Rigorous quality control and safety testing
Let’s discuss your needs—contact our team today for a consultation!
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- Is it safe to use lidocaine patches while breastfeeding? Expert Guidance for Nursing Mothers
- Are lidocaine patches safe to use during pregnancy? A Guide to Making an Informed Choice
- What systemic side effects can lidocaine patches cause? Minimizing Risks for Safe Pain Relief
- How can you use lidocaine patches for multiple sore spots? A Guide to Safe, Effective Pain Relief
- How are lidocaine patches typically used for pain relief during pregnancy? A Guide to Safe, Targeted Relief
















