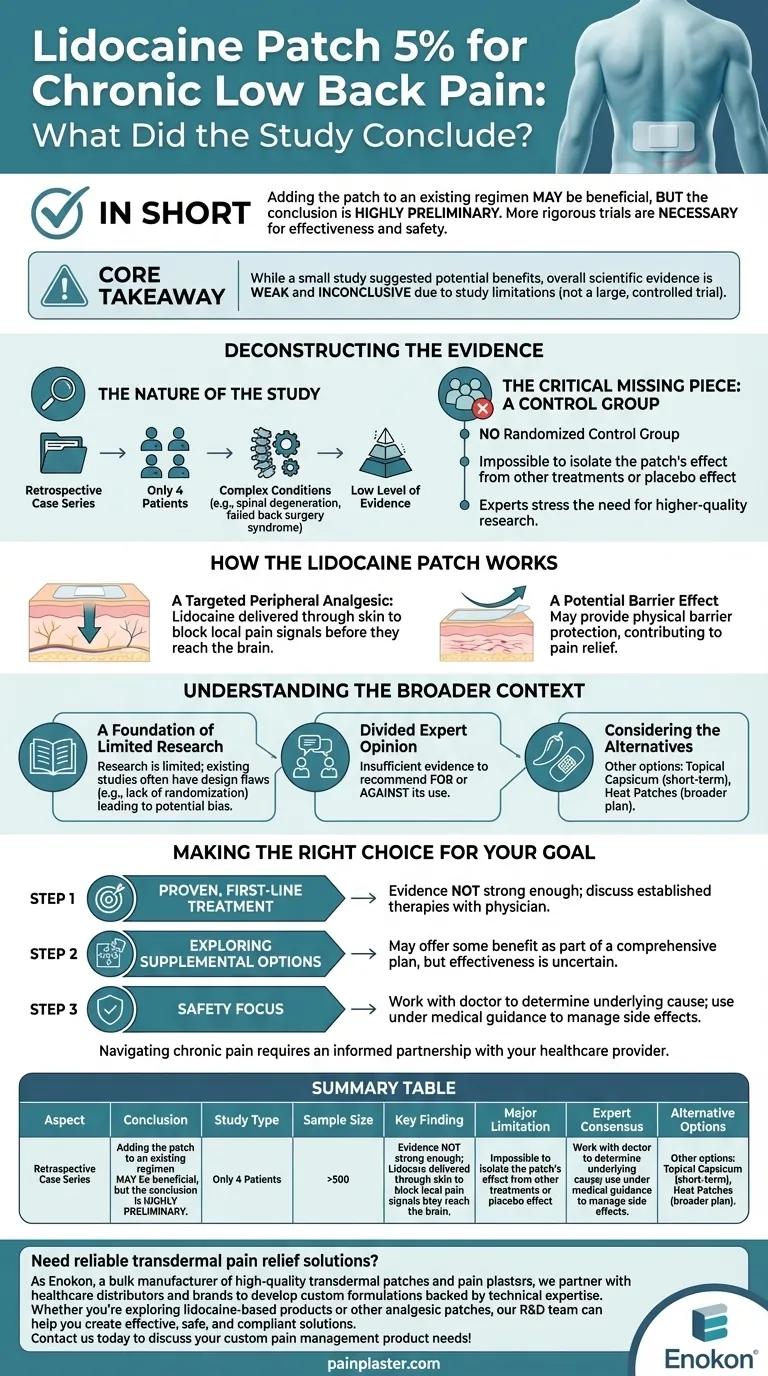
In short, the study concluded that adding the lidocaine patch 5% to an existing pain relief regimen may be beneficial for chronic low back pain. However, this conclusion was highly preliminary, and the authors stressed that more rigorous, controlled clinical trials are necessary to truly evaluate its effectiveness and safety.
The core takeaway is that while a small study suggested potential benefits, the overall scientific evidence supporting the lidocaine patch 5% for chronic low back pain is weak and inconclusive. The specific study was not a large, controlled trial, but a small retrospective case review, which limits the reliability of its findings.

Deconstructing the Evidence
To understand the study's conclusion, we must first look at the quality of the evidence it provides. The strength of a medical recommendation depends entirely on the strength of the research behind it.
The Nature of the Study
The investigation was a retrospective case series. This means researchers looked back at the records of just four patients who had already been treated.
These patients had complex conditions, including pain from spinal degeneration and complications from "failed back surgery syndrome."
This type of study can suggest a potential new use for a treatment, but it cannot prove that the treatment caused the improvement. It is considered a low level of medical evidence.
The Critical Missing Piece: A Control Group
The most significant limitation is the lack of a randomized control group.
Without a control group (a similar group of patients who did not receive the patch), it's impossible to know if the patients' improvement was due to the lidocaine patch, other treatments they were receiving, or simply a placebo effect.
This is why the study's own authors, and other experts, emphasize the need for higher-quality research.
How the Lidocaine Patch is Supposed to Work
Understanding the mechanism helps clarify its potential role and its limitations in treating chronic low back pain.
A Targeted Peripheral Analgesic
The patch delivers lidocaine, a common local anesthetic, directly through the skin to the painful area.
Its goal is to block or interrupt the local pain signals in the tissue before they can travel to the brain. This is different from oral pain medications that affect the entire body.
A Potential Barrier Effect
In studies for other conditions like shingles, researchers noted that the patch also provides a physical barrier. This can protect sensitive, irritated skin from friction, which may contribute to pain relief.
Understanding the Broader Context
The findings of one small study do not exist in a vacuum. The broader consensus among medical experts provides a more complete picture.
A Foundation of Limited Research
Multiple sources confirm that overall, research on lidocaine patches for chronic low back pain is limited. The few studies that exist often have design flaws, like the lack of randomization, that make their results potentially biased.
Divided Expert Opinion
Because the evidence is weak, experts are not in agreement. Some professional guidelines state there is insufficient evidence to either recommend for or against its use in treating chronic low back pain.
Considering the Alternatives
Other topical treatments are sometimes recommended instead. For short-term relief (up to three months), some experts suggest topical capsicum. Simple heat patches can also play a valuable role in a broader pain management plan.
Making the Right Choice for Your Goal
When considering any treatment, it is crucial to align the choice with your primary objective and discuss it with your healthcare provider.
- If your primary focus is a proven, first-line treatment: The evidence for the lidocaine patch is not strong enough; you should discuss more established therapies with your physician.
- If your primary focus is exploring supplemental options: The patch may offer some benefit as part of a comprehensive pain management plan, but its effectiveness is uncertain and should be monitored closely.
- If your primary focus is safety: You must work with a doctor to determine the underlying cause of your pain and use the patch only under medical guidance to manage potential side effects.
Ultimately, navigating chronic pain requires an informed partnership with your healthcare provider to build a comprehensive and effective treatment plan.
Summary Table:
| Aspect | Conclusion |
|---|---|
| Study Type | Retrospective case series (low evidence level) |
| Sample Size | 4 patients with complex conditions |
| Key Finding | Potential benefit as an add-on therapy, but not conclusive |
| Major Limitation | No control group; cannot rule out placebo effect |
| Expert Consensus | Insufficient evidence to recommend for or against use |
| Alternative Options | Topical capsicum, heat patches, established therapies |
Need reliable transdermal pain relief solutions? As Enokon, a bulk manufacturer of high-quality transdermal patches and pain plasters, we partner with healthcare distributors and brands to develop custom formulations backed by technical expertise. Whether you're exploring lidocaine-based products or other analgesic patches, our R&D team can help you create effective, safe, and compliant solutions. Contact us today to discuss your custom pain management product needs!
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- For what condition are lidocaine patches approved in the United Kingdom? A Guide to Postherpetic Neuralgia Treatment
- How is the lidocaine patch administered? A Step-by-Step Guide for Safe & Effective Pain Relief
- How does the lidocaine patch work? Targeted Relief for Nerve Pain Explained
- How should the treated area be protected while wearing a lidocaine patch? Safety Tips for Effective Pain Relief
- Is it safe to use lidocaine patches while breastfeeding? Expert Guidance for Nursing Mothers

















