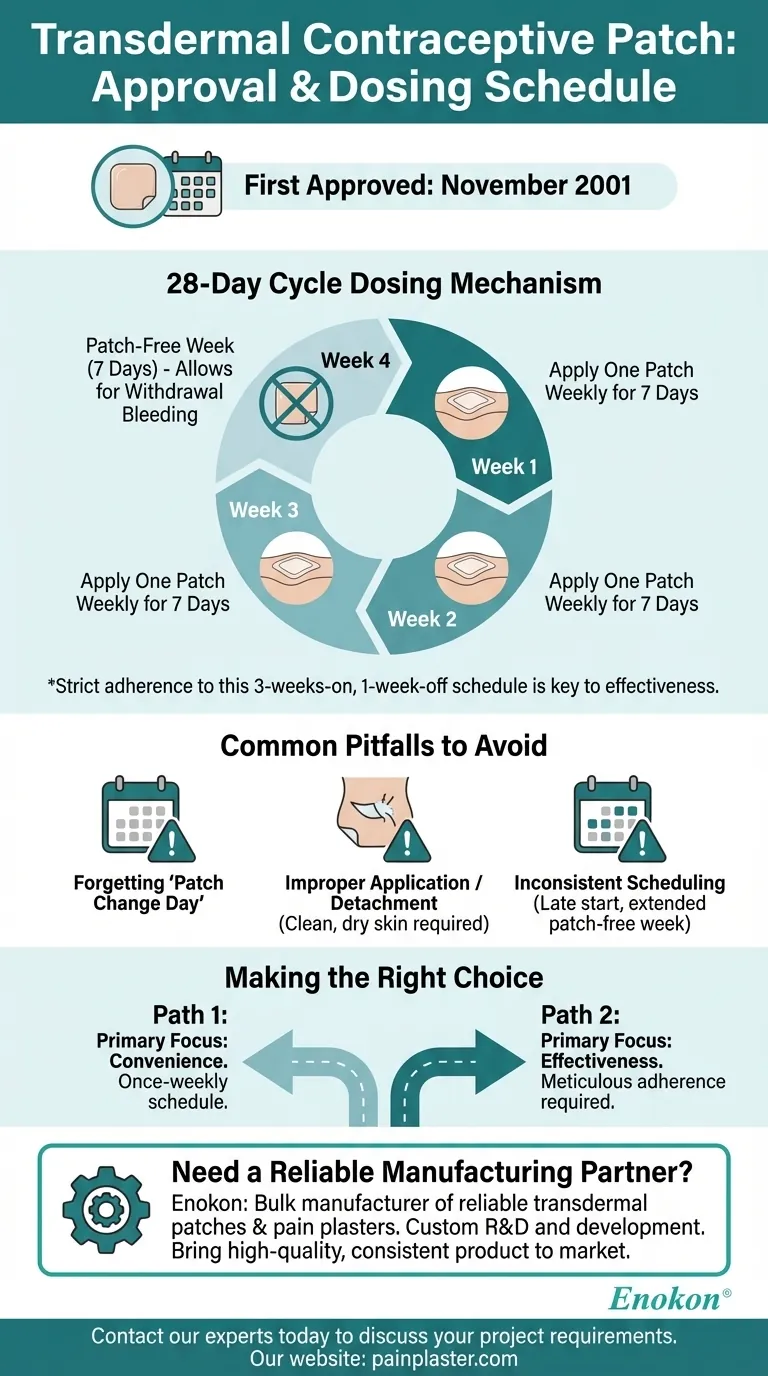
The first transdermal contraceptive patch was approved in November 2001. Its dosing is designed around a 28-day cycle, where one patch is applied to the skin and worn for seven days. This process is repeated with a new patch each week for three consecutive weeks, followed by one full week where no patch is worn.
The transdermal patch simplifies contraception by replacing a daily task with a weekly one. However, its effectiveness is entirely dependent on strict adherence to its distinct 28-day cycle: three weeks of continuous use followed by one patch-free week.

Understanding the Dosing Mechanism
The core principle behind the patch's schedule is to maintain consistent hormone levels in the body to prevent ovulation. The patch-free interval is designed to allow for withdrawal bleeding, which mimics a menstrual period.
The 28-Day Cycle Explained
A single contraceptive patch is worn for exactly seven days. It must be replaced on the same day of the week each time, which is known as the "Patch Change Day."
This weekly application is repeated for three straight weeks, for a total of 21 days of continuous hormone delivery.
The Purpose of the Patch-Free Week
Following the 21 days of patch use, the fourth week is a designated patch-free interval. No patch is worn for these seven days.
This break in hormone delivery causes the uterine lining to shed, resulting in withdrawal bleeding. A new cycle begins with a new patch application after the seven patch-free days are over.
Common Pitfalls to Avoid
While the weekly schedule is convenient, it introduces unique points of failure that require careful attention. Understanding these risks is critical for ensuring the patch's contraceptive efficacy.
Forgetting the 'Patch Change Day'
The most common error is forgetting to apply a new patch on the correct day. Delaying the start of a new patch after the patch-free week, or failing to change it on time during the three-week cycle, can compromise its effectiveness.
Improper Application or Detachment
The patch must be applied to clean, dry, intact skin. Lotions, oils, or creams can interfere with its adhesion. If a patch becomes partially or fully detached for an extended period, it may not deliver the necessary dose of hormones.
Inconsistent Scheduling
Starting a new patch cycle late or extending the patch-free week beyond seven days are significant risks. This can allow for ovulation to occur, increasing the chance of an unintended pregnancy.
Making the Right Choice for Your Goal
Adopting the transdermal patch requires a clear understanding of its cadence. The method's convenience is directly tied to the user's ability to adhere to its weekly schedule.
- If your primary focus is convenience: The patch's once-weekly schedule offers a significant advantage over methods requiring daily attention.
- If your primary focus is effectiveness: Success hinges on meticulously following the 3-weeks-on, 1-week-off schedule without deviation.
Ultimately, mastering this precise weekly cadence is the key to successfully using the transdermal patch as a reliable form of contraception.
Summary Table:
| Key Aspect | Details |
|---|---|
| Approval Date | November 2001 |
| Dosing Cycle | 28-day cycle |
| Active Use | 3 weeks (one patch applied weekly) |
| Patch-Free Interval | 1 week (allows for withdrawal bleeding) |
| Key to Effectiveness | Strict adherence to the weekly schedule |
Need a reliable manufacturing partner for transdermal patches?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we provide healthcare and pharma distributors and brands with the technical expertise for custom R&D and development. Let us help you bring a high-quality, consistent product to market.
Contact our experts today to discuss your project requirements.
Visual Guide
Related Products
- Prostate Pain Kidney Health Care Patch for Men
- Capsaicin Chili Medicated Pain Relief Patches
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
People Also Ask
- What should patients tell their doctor before using testosterone patches? A Guide to Safe Treatment
- What should be done if a dose of testosterone patches is missed? Regain Stability and Safety
- What should be done if a testosterone patch falls off? A Guide to Maintaining Hormone Stability
- What lifestyle factors should be considered when choosing between testosterone patches and injections? Find Your Best Fit
- What should be done in case of a testosterone patch overdose? A Step-by-Step Emergency Guide














