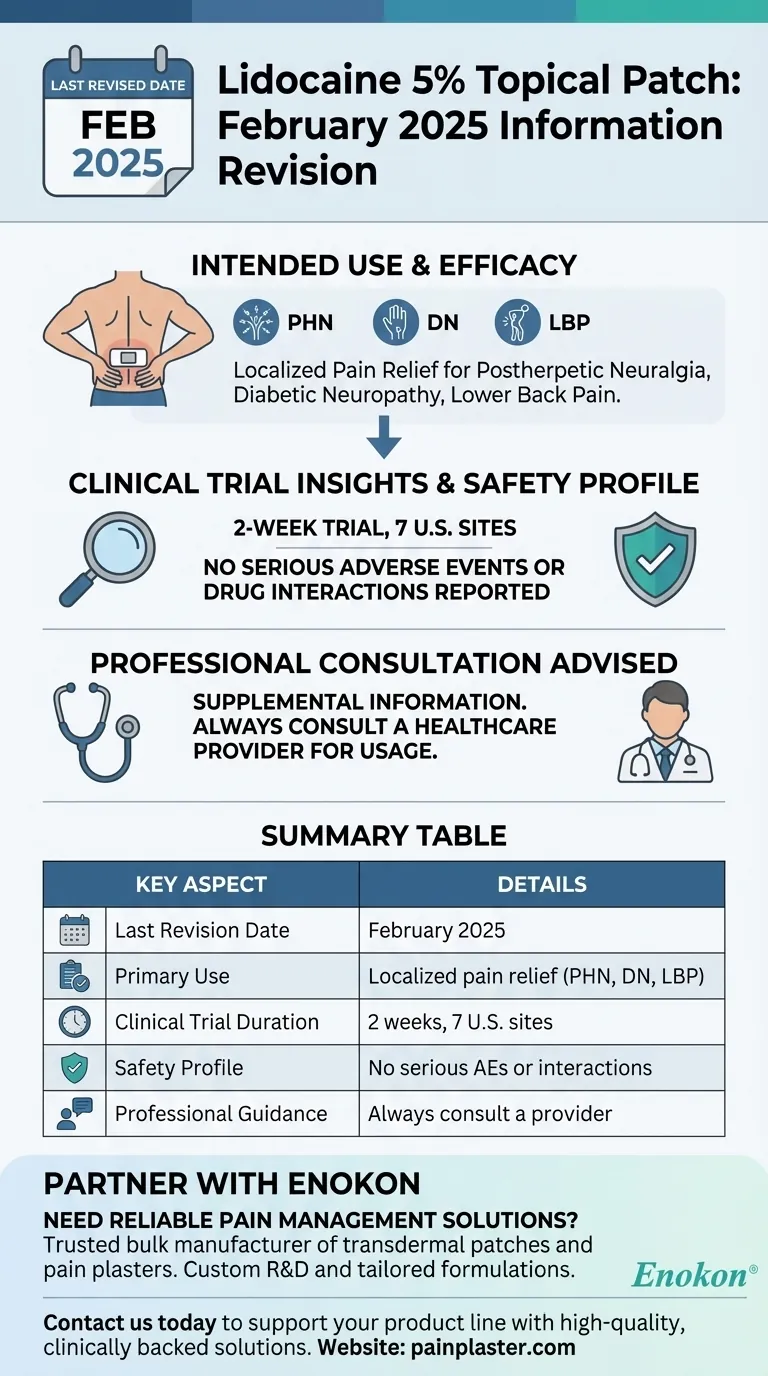
The information about the lidocaine patch 5 percent was last revised in February 2025. This update ensures that users have access to the most current data regarding its efficacy, safety, and usage guidelines. The patch is designed for localized pain relief, particularly for conditions like postherpetic neuralgia (PHN), painful diabetic neuropathy (DN), and lower back pain (LBP). However, it is crucial to consult a healthcare professional before use, as the information provided is supplementary and not a replacement for medical advice.
Key Points Explained:
-
Revision Date
- The most recent update to the information about the lidocaine 5% topical patch was in February 2025. This revision reflects the latest clinical findings and usage recommendations.
-
Intended Use and Efficacy
- The patch is effective in reducing moderate-to-severe chronic pain associated with PHN, DN, or LBP.
- It targets localized pain and is well-tolerated when used alongside other analgesic treatments, with no reported serious adverse effects or drug interactions.
-
Clinical Trial Insights
- The supporting study was an open-label, non-randomized trial conducted over 2 weeks across 7 U.S. sites.
- Patients applied up to 4 patches every 24 hours to the area of maximal pain while maintaining their existing pain management regimens.
-
Professional Consultation Advised
- The information is supplemental and should not replace professional medical judgment.
- Specific usage instructions (e.g., application frequency, duration) must be discussed with a healthcare provider to ensure safe and effective use.
-
Safety Profile
- No serious adverse events (AEs) or harmful drug interactions were reported in the trial, highlighting its compatibility with other analgesics.
For optimal outcomes, always combine this information with guidance from a healthcare professional. The patch’s role in pain management underscores the importance of personalized medical advice in chronic care.
Summary Table:
| Key Aspect | Details |
|---|---|
| Last Revision Date | February 2025 |
| Primary Use | Localized pain relief (PHN, DN, LBP) |
| Clinical Trial Duration | 2 weeks, 7 U.S. sites |
| Safety Profile | No serious adverse events or drug interactions reported |
| Professional Guidance | Always consult a healthcare provider for personalized usage instructions |
Need reliable pain management solutions? Partner with Enokon, a trusted bulk manufacturer of transdermal patches and pain plasters for healthcare distributors and brands. Our expertise in custom R&D ensures tailored formulations for your needs. Contact us today to discuss how we can support your product line with high-quality, clinically backed solutions.
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
People Also Ask
- For what condition are lidocaine patches approved in the United Kingdom? A Guide to Postherpetic Neuralgia Treatment
- How should the treated area be protected while wearing a lidocaine patch? Safety Tips for Effective Pain Relief
- How are lidocaine patches typically used for pain relief during pregnancy? A Guide to Safe, Targeted Relief
- How is the lidocaine patch administered? A Step-by-Step Guide for Safe & Effective Pain Relief
- Are lidocaine patches safe to use during pregnancy? A Guide to Making an Informed Choice














