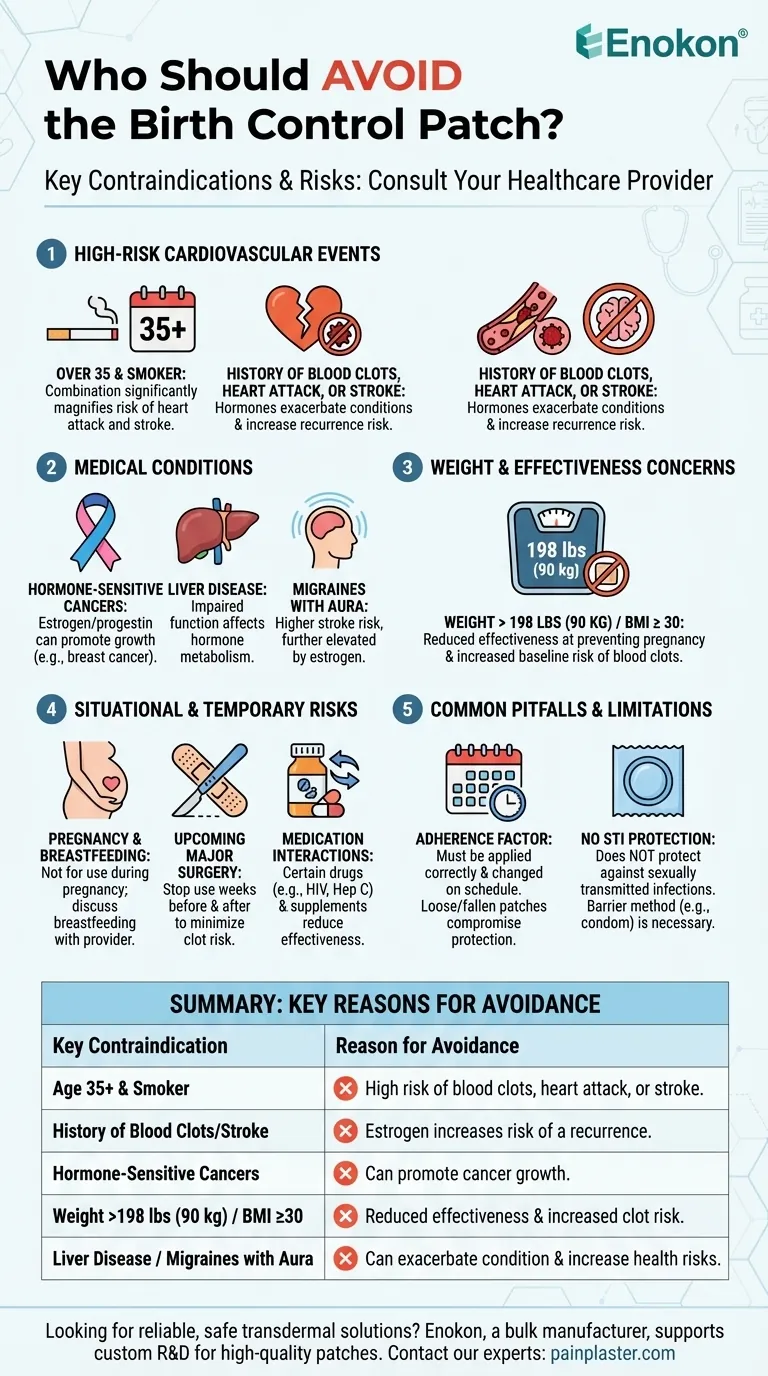
The birth control patch is not a safe or effective option for everyone. Specifically, you should avoid using it if you are over 35 and smoke, have a history of blood clots, heart attack, or stroke, have certain hormone-sensitive cancers, or have a body weight over 198 pounds (90 kg) or a BMI of 30 or more. A range of other medical conditions, such as liver disease, uncontrolled high blood pressure, and migraines with aura, also make the patch a poor choice.
The core reason for most of these restrictions is risk. The estrogen in the patch increases the risk of serious cardiovascular events like blood clots, heart attacks, and strokes, especially when combined with other risk factors like age, smoking, or a history of vascular disease.

Key Risk Factors and Contraindications
Understanding why certain conditions are contraindications is essential for making an informed health decision. The primary concerns revolve around cardiovascular health, hormone sensitivity, and effectiveness.
Age and Smoking
For individuals over 35 who smoke, the combination is particularly dangerous. Smoking and the estrogen in the patch both independently increase the risk of blood clots. When used together, this risk is magnified significantly, elevating the chances of a heart attack or stroke.
Cardiovascular and Blood Clot History
If you have a personal history of blood clots (deep vein thrombosis or pulmonary embolism), heart attack, stroke, or uncontrolled high blood pressure, the patch is not for you. The hormones can exacerbate these conditions and increase the likelihood of a recurrence.
Certain Cancers
The patch contains estrogen and progestin, which can promote the growth of hormone-sensitive cancers. For this reason, it is contraindicated for individuals with a history of certain cancers, such as breast cancer.
Body Weight and BMI
Effectiveness and safety are a concern for individuals with a higher body weight. The patch may be less effective at preventing pregnancy in those who weigh more than 198 pounds (90 kg). Additionally, a BMI of 30 or more is a contraindication due to an increased baseline risk of blood clots, which the patch can further elevate.
Other Significant Medical Conditions
Several other health issues make the patch an unsafe option:
- Liver Disease: The liver metabolizes the hormones from the patch, and impaired function can be dangerous.
- Migraines with Aura: This type of migraine is associated with a higher risk of stroke, a risk that is further increased by the patch's estrogen.
- Diabetes with Complications: If diabetes has already caused damage to your blood vessels, the patch can add to the cardiovascular strain.
- Unexplained Vaginal Bleeding: This should be diagnosed by a doctor before starting any hormonal birth control to rule out a serious underlying condition.
Situational and Temporary Considerations
Beyond chronic conditions, some temporary situations make the patch unsuitable.
Pregnancy and Breastfeeding
The patch should not be used if you are pregnant. If you are breastfeeding, you should discuss the risks with your provider, as it is not known for certain if the hormones pass into breast milk and what effect they might have.
Upcoming Major Surgery
Surgery and the subsequent recovery period increase your risk of developing blood clots. Your doctor will likely advise you to stop using the patch for several weeks before and after a major surgical procedure to minimize this risk.
Medication Interactions
The patch’s effectiveness can be reduced by certain other medications. These include specific drugs used to treat HIV or Hepatitis C, as well as some supplements. Always disclose all medications you are taking to your healthcare provider.
Common Pitfalls to Avoid
Even for suitable candidates, the patch's success depends on correct usage and understanding its limitations.
The Adherence Factor
The patch must be applied correctly to clean, dry skin and changed on schedule every week. You should check it daily to ensure it is sticking properly. If it becomes loose or falls off, its contraceptive protection may be compromised.
The Weight Limitation
It is crucial to be honest about your weight with your provider. The 198-pound (90 kg) threshold is a key factor in the patch's reliability, and exceeding it means you should explore other, more effective contraceptive options.
No Protection Against STIs
Like all hormonal contraceptives, the birth control patch does not protect against sexually transmitted infections (STIs). A barrier method, like a condom, is still necessary for STI protection.
Making the Right Choice for Your Health
Selecting a contraceptive method requires a clear understanding of your personal health profile.
- If your primary focus is convenience and you are a smoker over 35: The patch is not a safe option due to the high risk of serious cardiovascular events.
- If you have a history of blood clots, stroke, or certain heart conditions: The patch is contraindicated because it can significantly increase your risk of another event.
- If your weight is over 198 lbs (90 kg) or your BMI is 30 or more: The patch is not recommended as it may not be effective at preventing pregnancy.
- If you have complex medical conditions like migraines with aura or liver disease: These conditions make the patch an unsafe choice, and you must discuss alternatives with your doctor.
Ultimately, a thorough and honest conversation with your healthcare provider is the only way to determine the safest and most effective birth control method for you.
Summary Table:
| Key Contraindication | Reason for Avoidance |
|---|---|
| Age 35+ & Smoker | High risk of blood clots, heart attack, or stroke. |
| History of Blood Clots/Stroke | Estrogen increases risk of a recurrence. |
| Hormone-Sensitive Cancers | Can promote cancer growth. |
| Weight >198 lbs (90 kg) / BMI ≥30 | Reduced effectiveness and increased clot risk. |
| Liver Disease / Migraines with Aura | Can exacerbate the condition and increase health risks. |
Looking for a reliable, safe transdermal solution for your patients or brand? As Enokon, a bulk manufacturer of high-quality transdermal patches and pain plasters, we understand the critical importance of safety and efficacy. Our technical expertise supports custom R&D to meet your specific needs. Contact our experts today to discuss how we can partner to develop effective and reliable transdermal products for the healthcare and pharmaceutical markets.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Prostate Pain Kidney Health Care Patch for Men
- Menthol Gel Pain Relief Patch
- Capsaicin Chili Medicated Pain Relief Patches
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
People Also Ask
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism

















