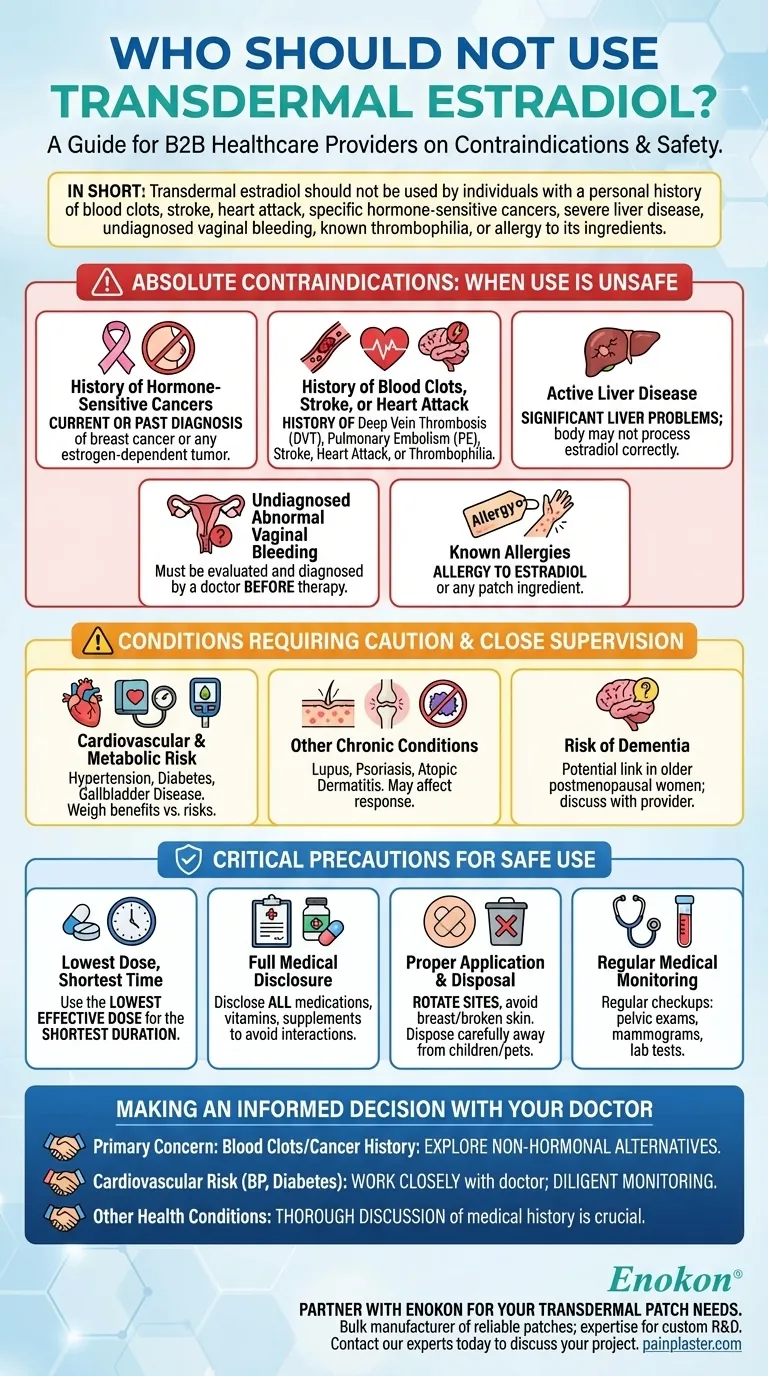
In short, transdermal estradiol should not be used by individuals with a personal history of blood clots, stroke, heart attack, or specific hormone-sensitive cancers like breast cancer. It is also contraindicated for those with severe liver disease, undiagnosed vaginal bleeding, known thrombophilia (clotting disorders), or a known allergy to any of its ingredients.
The decision to use transdermal estradiol hinges on a careful evaluation of your personal and family medical history. While it is highly effective for menopausal symptoms, its use is absolutely contraindicated for anyone with a history of estrogen-driven cancers or significant thromboembolic events.

Absolute Contraindications: When Use is Unsafe
Certain medical conditions create a risk profile where the potential harms of transdermal estradiol outweigh the benefits. If you have any of the following, this medication is generally not considered a safe option.
History of Hormone-Sensitive Cancers
Estrogen can stimulate the growth of certain cancers. For this reason, transdermal estradiol is contraindicated if you have a current or past diagnosis of breast cancer or any other estrogen-dependent tumor.
History of Blood Clots, Stroke, or Heart Attack
Estrogen therapy can increase the risk of blood clot formation. Therefore, it should not be used by individuals with a history of deep vein thrombosis (DVT), pulmonary embolism (PE), stroke, or heart attack. This also applies to those with known blood clotting disorders (thrombophilia).
Active Liver Disease
The liver is responsible for metabolizing hormones. If you have significant liver problems or disease, your body may not be able to process the estradiol correctly, leading to unsafe levels in your system.
Undiagnosed Abnormal Vaginal Bleeding
Abnormal bleeding can be a symptom of a serious underlying condition, such as uterine cancer. It is essential to have this evaluated and diagnosed by a doctor before considering any hormone therapy.
Known Allergies
A known allergy to estradiol or any other ingredient in the patch, such as the adhesives, is a direct contraindication.
Conditions Requiring Caution and Close Supervision
Beyond the absolute contraindications, many other conditions require a careful discussion with your doctor. In these cases, the medication may still be an option, but it necessitates closer monitoring.
Cardiovascular and Metabolic Risk Factors
Conditions like hypertension (high blood pressure), diabetes, and gallbladder disease can be influenced by hormone therapy. Your doctor will need to weigh the benefits against the potential for increased risk.
Other Chronic Conditions
Inform your doctor if you have conditions like lupus, psoriasis, or atopic dermatitis. These may affect how your body responds to the medication or how your skin reacts to the patch.
Risk of Dementia
Some studies have shown a link between estrogen therapy and dementia in older postmenopausal women. This potential risk should be part of the conversation with your healthcare provider.
Critical Precautions for Safe Use
If you and your doctor decide transdermal estradiol is appropriate, adhering to specific safety protocols is essential.
The "Lowest Dose, Shortest Time" Principle
The guiding principle for all hormone therapy is to use the lowest effective dose for the shortest duration necessary to manage your symptoms.
Full Medical Disclosure
Your doctor must have a complete picture of your health. Disclose all medications, vitamins, supplements, and herbal products you are taking, as they can interact with estradiol.
Proper Application and Disposal
Always rotate the application site on your skin to prevent irritation and avoid applying patches to the breasts or to broken skin. Used patches still contain medication and must be disposed of carefully, away from children and pets.
Regular Medical Monitoring
While on this therapy, you will need regular medical checkups, which may include pelvic exams, mammograms, and lab tests to monitor your body's response and ensure the treatment remains safe and effective for you.
Making an Informed Decision with Your Doctor
The choice to use transdermal estradiol is a collaborative one made between you and your healthcare provider, based on your unique health profile.
- If your primary concern is a history of blood clots or hormone-sensitive cancer: This medication is almost certainly not a safe option, and you must explore non-hormonal alternatives.
- If you have cardiovascular risk factors like high blood pressure or diabetes: You must work closely with your doctor to weigh the treatment benefits against the increased risks, requiring diligent monitoring.
- If you have no major contraindications but have other health conditions: A thorough discussion about your entire medical history is crucial to ensure this therapy is appropriate and safe for you.
Ultimately, a transparent conversation with your doctor is the only way to determine if transdermal estradiol is the right choice for your health goals.
Summary Table:
| Contraindication Group | Key Conditions |
|---|---|
| Absolute Contraindications | History of blood clots (DVT/PE), stroke, heart attack, hormone-sensitive cancers (e.g., breast cancer), severe liver disease, undiagnosed vaginal bleeding, known allergy. |
| Conditions Requiring Caution | Hypertension, diabetes, gallbladder disease, lupus, psoriasis, dementia risk. |
Partner with Enokon for Your Transdermal Patch Needs
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharmaceutical distributors and brands with the technical expertise for custom R&D and development. Ensure your hormone therapy products are safe, effective, and tailored to specific patient contraindications.
Contact our experts today to discuss your custom transdermal patch project.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
- Heating Pain Relief Patches for Menstrual Cramps
People Also Ask
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
















