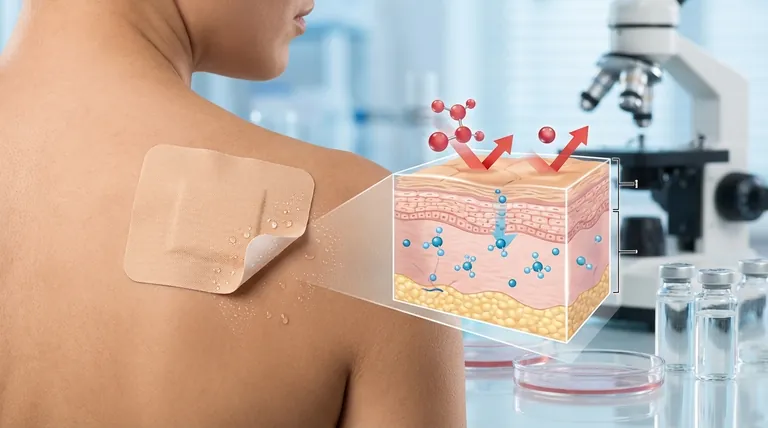
Transdermal patches are designed to deliver medication through the skin into the bloodstream, but their effectiveness often depends on the properties of the active ingredients and the formulation. Many commercially available patches fail because they contain molecules that are either too large or not fat-soluble, which prevents efficient absorption through the skin's barrier. The skin's outermost layer, the stratum corneum, is highly selective, allowing only small, lipophilic (fat-soluble) molecules to pass through. If the drug doesn't meet these criteria, absorption is minimal, rendering the patch ineffective. Additionally, formulation issues such as poor adhesive properties or incorrect dosage can further reduce efficacy.
Key Points Explained:
-
Molecular Size and Solubility
- The skin's stratum corneum acts as a selective barrier, primarily allowing small (typically < 500 Daltons) and fat-soluble molecules to penetrate.
- Many drugs in transdermal patches fail because they are either too large or hydrophilic (water-soluble), preventing efficient absorption.
- Example: Large peptides or polar molecules may remain trapped in the patch or skin surface, never reaching systemic circulation.
-
Formulation Challenges
- Even if a drug has suitable properties, poor patch design can hinder delivery.
- Issues include:
- Insufficient drug concentration in the adhesive layer.
- Ineffective penetration enhancers (chemicals that temporarily disrupt the skin barrier).
- Unstable drug formulations that degrade before absorption.
-
Skin Variability
- Absorption rates vary based on skin thickness, hydration, and individual metabolism.
- Patches tested on idealized lab conditions may underperform in real-world use due to these factors.
-
Adhesion and Wear Time
- Poor adhesive quality can cause patches to detach prematurely, reducing drug exposure.
- Sweat, movement, or skin oils may further compromise adhesion.
-
Regulatory and Commercial Pressures
- Some patches are marketed despite limited efficacy due to:
- Lower development costs compared to oral or injectable alternatives.
- Consumer demand for non-invasive options, even if performance is suboptimal.
- Some patches are marketed despite limited efficacy due to:
-
Alternatives and Solutions
- Advanced technologies (e.g., microneedle patches) are emerging to bypass the stratum corneum.
- Reformulating drugs with better penetration enhancers or nano-carriers could improve outcomes.
For purchasers, evaluating patches requires scrutiny of:
- Drug properties (size, lipophilicity).
- Clinical trial data showing measurable absorption.
- Manufacturer reputation for quality control.
The gap between theory and real-world performance underscores why many patches disappoint—highlighting the need for better design and transparency in the industry.
Summary Table:
| Key Issue | Impact on Patch Efficacy |
|---|---|
| Large/Hydrophilic Drugs | Poor absorption due to skin barrier selectivity (needs small, fat-soluble molecules). |
| Weak Formulation | Low drug concentration, unstable compounds, or ineffective enhancers reduce delivery. |
| Skin Variability | Thickness, hydration, and metabolism differences lead to inconsistent results. |
| Adhesion Failures | Patches detach prematurely, cutting off drug exposure. |
| Regulatory Gaps | Some patches prioritize cost/consumer demand over proven efficacy. |
Need high-performance transdermal patches? Partner with Enokon—a trusted bulk manufacturer of reliable transdermal patches and pain plasters for healthcare distributors and brands. Our expertise in custom R&D ensures optimal drug delivery through scientifically validated formulations. Contact us today to discuss tailored solutions for your needs!
Visual Guide

Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Herbal Eye Protection Patch Eye Patch
- Heating Pain Relief Patches for Menstrual Cramps
- Menthol Gel Pain Relief Patch
People Also Ask
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained















