In short, you must consult your healthcare provider before using lidocaine patches during pregnancy or while breastfeeding. While they are often considered a potential option due to low systemic absorption, robust safety data is limited. A doctor must evaluate your specific situation to determine if the benefits of targeted pain relief outweigh any potential risks.
The core issue isn't whether lidocaine patches are "good" or "bad," but rather how much of the medication enters your bloodstream. Because the patches work locally, very little of the drug is absorbed systemically, making them a potential option when oral medications are best avoided—but only with a doctor's explicit approval.

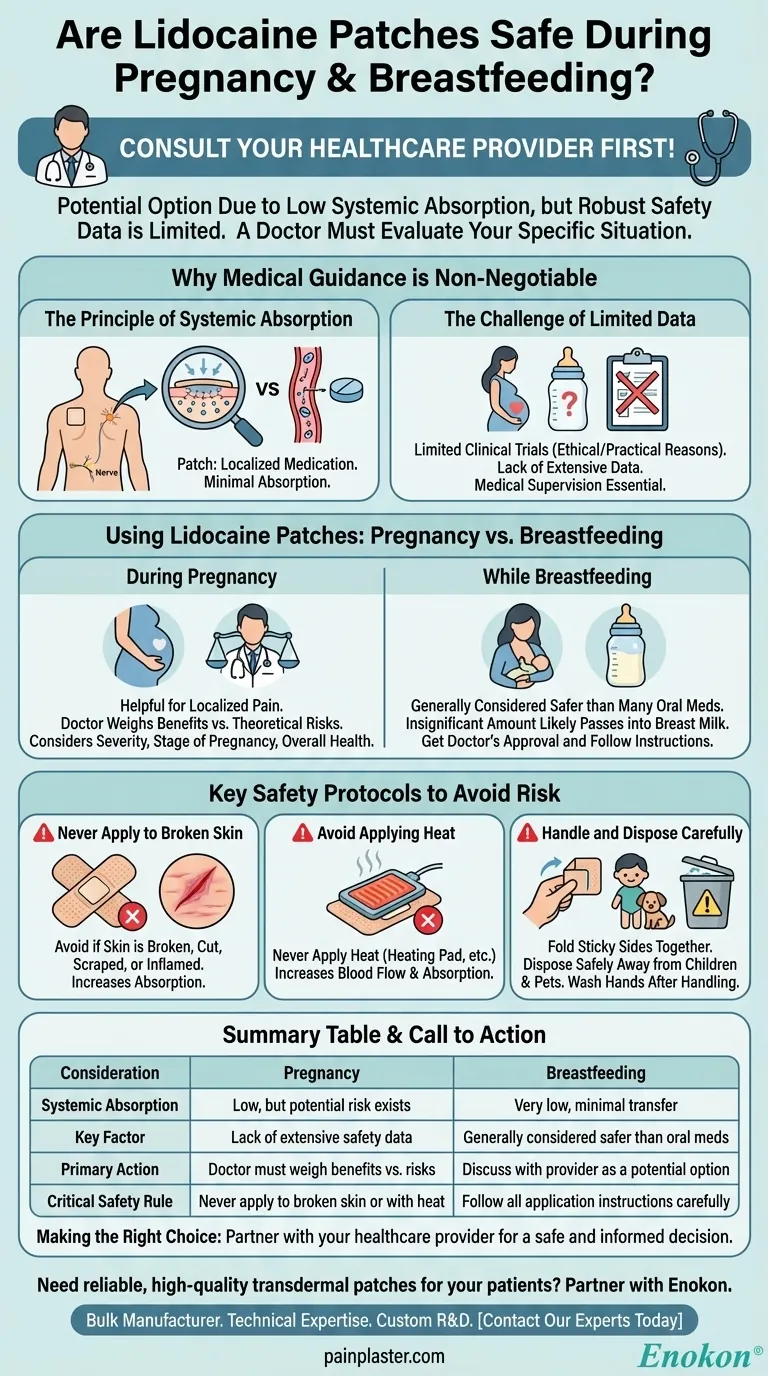
Why Medical Guidance is Non-Negotiable
The caution around any medication during pregnancy and breastfeeding stems from one central concern: potential effects on the baby. Lidocaine patches offer an advantage over pills because they are designed to work directly where you apply them.
The Principle of Systemic Absorption
A lidocaine patch delivers medication directly to the nerve endings in a specific area of your skin.
Unlike an oral painkiller that travels through your entire bloodstream to work, a patch keeps the medication localized. This means the amount that gets absorbed into your system—and could potentially reach a baby—is minimal.
The Challenge of Limited Data
Ethical and practical reasons limit the number of clinical trials conducted on pregnant individuals.
Because of this, there is not extensive data to definitively prove absolute safety. This lack of data is why medical supervision is not just recommended; it is essential.
Using Lidocaine Patches: Pregnancy vs. Breastfeeding
While the core principle of low absorption applies to both situations, the considerations are slightly different.
During Pregnancy
When used as directed under medical supervision, lidocaine patches can be a helpful tool for localized pain.
Your doctor will weigh the benefits of managing your pain against the theoretical risks. They will consider the location and severity of your pain, the stage of your pregnancy, and your overall health profile before making a recommendation.
While Breastfeeding
Lidocaine patches are generally considered a safer choice for pain relief while breastfeeding compared to many oral medications.
The low rate of systemic absorption means that an insignificant amount of the drug is likely to pass into breast milk. However, it is still critical to get a doctor's approval and follow all application instructions carefully.
Key Safety Protocols to Avoid Risk
If your doctor approves the use of a lidocaine patch, following safety guidelines is paramount to minimize risk and ensure effectiveness.
Never Apply to Broken Skin
Do not apply patches to skin that is broken, cut, scraped, or inflamed. This can significantly increase the amount of medication absorbed into your bloodstream.
Avoid Applying Heat
Never apply a heating pad or any other heat source over a lidocaine patch. Heat increases blood flow to the area, which can cause your body to absorb the medication much more rapidly than intended.
Handle and Dispose of Patches Carefully
Keep new and used patches away from the eyes, and wash your hands immediately after handling them. Used patches still contain active medication, so fold them in half with the sticky sides together and dispose of them safely away from children and pets.
Making the Right Choice for Your Goal
Your path forward depends on balancing your need for pain relief with the priority of protecting your child.
- If your primary focus is managing pain during pregnancy: Your first and only step is to speak with your doctor. They can determine if a lidocaine patch is an appropriate choice for your specific circumstances.
- If your primary focus is safe pain relief while breastfeeding: Discuss lidocaine patches with your provider as a likely safer alternative to systemic pain medications, ensuring you understand all application and safety rules.
Ultimately, partnering with your healthcare provider is the only way to ensure you are making a safe and informed decision for both you and your baby.
Summary Table:
| Consideration | Pregnancy | Breastfeeding |
|---|---|---|
| Systemic Absorption | Low, but potential risk exists | Very low, minimal transfer to breast milk |
| Key Factor | Lack of extensive safety data | Generally considered safer than oral meds |
| Primary Action | Doctor must weigh benefits vs. risks | Discuss with provider as a potential option |
| Critical Safety Rule | Never apply to broken skin or with heat | Follow all application instructions carefully |
Need reliable, high-quality transdermal patches for your patients? Partner with Enokon, a bulk manufacturer of trusted lidocaine patches and pain relief plasters for healthcare distributors and pharmaceutical brands. Our technical expertise ensures product consistency and safety. Let us support your custom R&D and development needs to deliver effective pain management solutions. Contact our experts today to discuss your requirements.
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Medical Cooling Gel Patches for Fever Cooling Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
People Also Ask
- How is the lidocaine patch administered? A Step-by-Step Guide for Safe & Effective Pain Relief
- How are lidocaine patches typically used for pain relief during pregnancy? A Guide to Safe, Targeted Relief
- When should someone contact a doctor regarding lidocaine patch use? Ensure Safe Pain Relief
- How can you use lidocaine patches for multiple sore spots? A Guide to Safe, Effective Pain Relief
- Is it safe to use lidocaine patches while breastfeeding? Expert Guidance for Nursing Mothers
















