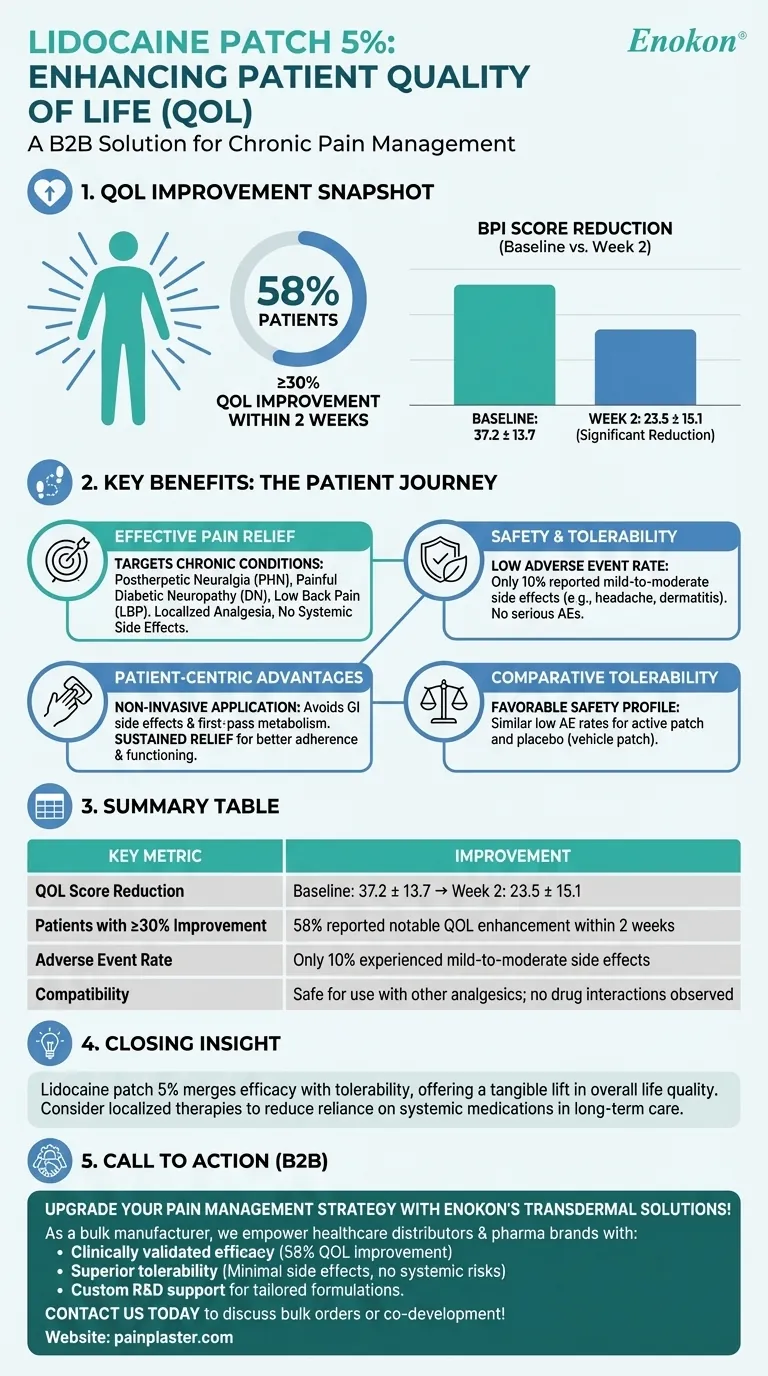
The lidocaine patch 5% (lidocaine patch 5 percent) demonstrated a significant positive impact on patients' quality of life (QOL), particularly in those with moderate-to-severe chronic pain conditions like postherpetic neuralgia (PHN), painful diabetic neuropathy (DN), or low back pain (LBP). Clinical data showed a notable reduction in pain-related QOL impairment, with 58% of patients achieving at least a 30% improvement in QOL scores within two weeks. The patch was well-tolerated, with minimal adverse effects, and could be safely combined with other pain management therapies.

Key Points Explained:
1. Improvement in Quality of Life (QOL) Metrics
- Baseline to Week 2 Reduction: The BPI (Brief Pain Inventory) composite score for QOL impairment decreased from 37.2 ± 13.7 at baseline to 23.5 ± 15.1 by Week 2, indicating a statistically significant improvement.
- Patient-Reported Benefits: 58% of patients experienced at least a 30% improvement in QOL scores, highlighting the patch's efficacy in alleviating pain-related life disruptions.
2. Effective Pain Relief Across Conditions
- The patch reduced the intensity of common pain qualities in chronic conditions such as:
- Postherpetic neuralgia (PHN)
- Painful diabetic neuropathy (DN)
- Low back pain (LBP)
- Its mechanism of localized analgesia allowed for targeted relief without systemic side effects common in oral medications.
3. Safety and Tolerability
- Low Adverse Event Rate: Only 10% of patients reported mild-to-moderate side effects (e.g., headache, dermatitis, taste disturbance). No serious adverse events (AEs) or clinically significant changes in vital signs/lab values were observed.
- Compatibility with Other Therapies: The patch could be safely used alongside other analgesics without adverse drug interactions, making it a versatile option for multimodal pain management.
4. Patient-Centric Advantages
- Non-Invasive Application: Unlike oral medications, the patch avoids gastrointestinal side effects and first-pass metabolism.
- Sustained Relief: Continuous drug delivery improved adherence and consistency in pain control, indirectly enhancing daily functioning and emotional well-being.
5. Comparative Tolerability
- Both the active lidocaine patch and the placebo (vehicle patch) had similarly low AE rates, reinforcing its favorable safety profile.
Closing Insight
The lidocaine patch 5% represents a practical advancement in chronic pain management, merging efficacy with tolerability. For patients burdened by persistent pain, it offers not just symptom relief but a tangible lift in overall life quality—proving that sometimes, the smallest interventions deliver the most profound changes. Have you considered how localized therapies like this could reduce reliance on systemic medications in long-term care?
Summary Table:
| Key Metric | Improvement |
|---|---|
| QOL Score Reduction | Baseline: 37.2 ± 13.7 → Week 2: 23.5 ± 15.1 (statistically significant) |
| Patients with ≥30% Improvement | 58% reported notable QOL enhancement within 2 weeks |
| Adverse Event Rate | Only 10% experienced mild-to-moderate side effects (e.g., headache, dermatitis) |
| Compatibility | Safe for use with other analgesics; no drug interactions observed |
Upgrade your pain management strategy with Enokon’s transdermal solutions!
As a bulk manufacturer of reliable lidocaine patches and custom pain relief plasters, we empower healthcare distributors and pharma brands with:
- Clinically validated efficacy (58% QOL improvement in chronic pain patients)
- Superior tolerability (minimal side effects, no systemic risks)
- Custom R&D support for tailored formulations
Contact us today to discuss bulk orders or co-development of patient-centric pain therapies!
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How can you use lidocaine patches for multiple sore spots? A Guide to Safe, Effective Pain Relief
- Are lidocaine patches safe to use during pregnancy? A Guide to Making an Informed Choice
- How does the lidocaine patch work? Targeted Relief for Nerve Pain Explained
- How are lidocaine patches typically used for pain relief during pregnancy? A Guide to Safe, Targeted Relief
- For what condition are lidocaine patches approved in the United Kingdom? A Guide to Postherpetic Neuralgia Treatment
















