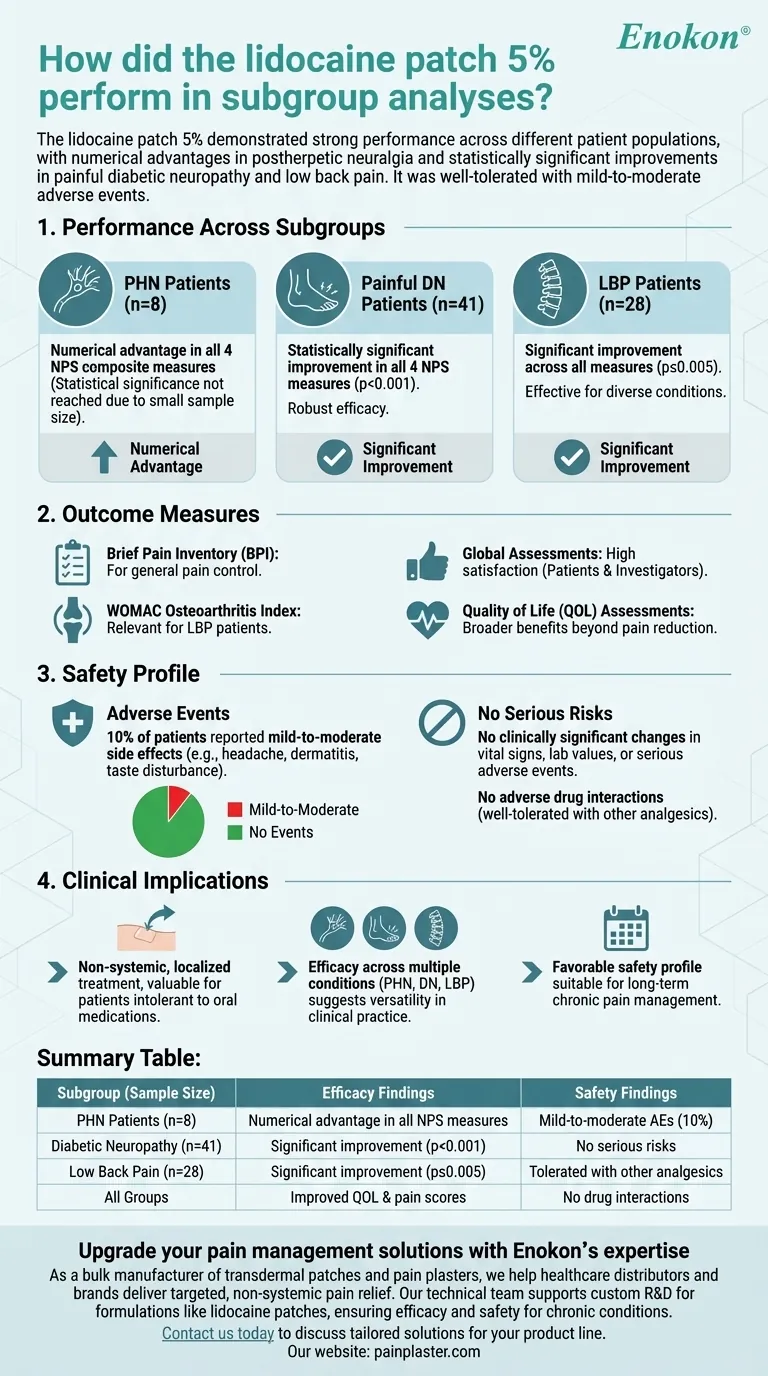
The lidocaine patch 5% demonstrated strong performance in subgroup analyses across different patient populations. It showed a numerical advantage in all four Neuropathic Pain Scale (NPS) composite measures for postherpetic neuralgia (PHN) patients (n=8) and statistically significant improvements in painful diabetic neuropathy (DN) (n=41; p<0.001) and low back pain (LBP) patients (n=28; p≤0.005). The patch was effective in reducing pain intensity across various chronic pain conditions and was well-tolerated, with only mild-to-moderate adverse events reported in 10% of patients. Outcome measures included multiple validated pain and quality of life assessments, showing comprehensive benefits.
Key Points Explained:
-
Performance Across Subgroups:
- PHN Patients (n=8): Showed numerical advantage in all four NPS composite measures, though statistical significance wasn't reached due to small sample size.
- Painful DN Patients (n=41): Achieved statistically significant improvement in all four NPS measures (p<0.001), indicating robust efficacy.
- LBP Patients (n=28): Also showed significant improvement across all measures (p≤0.005), reinforcing its effectiveness for diverse pain conditions.
-
Outcome Measures:
- The study used multiple validated tools to assess efficacy:
- Brief Pain Inventory (BPI): For general pain control.
- WOMAC Osteoarthritis Index: Particularly relevant for LBP patients.
- Global Assessments: Both patients and investigators reported high satisfaction with the lidocaine patch 5 percent.
- Quality of Life (QOL) Assessments: Demonstrated broader benefits beyond pain reduction.
- The study used multiple validated tools to assess efficacy:
-
Safety Profile:
- Adverse Events: 10% of patients experienced mild-to-moderate side effects (e.g., headache, dermatitis, taste disturbance).
- No Serious Risks: No clinically significant changes in vital signs, lab values, or serious adverse events were reported.
- Combination Therapy: The patch was well-tolerated even when used with other analgesics, with no adverse drug interactions.
-
Clinical Implications:
- The patch provides a non-systemic, localized treatment option for chronic pain, which is particularly valuable for patients who cannot tolerate oral medications.
- Its efficacy across multiple pain conditions (PHN, DN, LBP) suggests versatility in clinical practice.
- The favorable safety profile makes it suitable for long-term use in chronic pain management.
Have you considered how such targeted topical therapies could reduce reliance on systemic pain medications in your practice? These patches represent one of many technologies quietly transforming modern pain management.
Summary Table:
| Subgroup (Sample Size) | Efficacy Findings | Safety Findings |
|---|---|---|
| PHN Patients (n=8) | Numerical advantage in all NPS measures | Mild-to-moderate AEs (10%) |
| Diabetic Neuropathy (n=41) | Significant improvement (p<0.001) | No serious risks |
| Low Back Pain (n=28) | Significant improvement (p≤0.005) | Tolerated with other analgesics |
| All Groups | Improved QOL & pain scores | No drug interactions |
Upgrade your pain management solutions with Enokon’s expertise
As a bulk manufacturer of transdermal patches and pain plasters, we help healthcare distributors and brands deliver targeted, non-systemic pain relief. Our technical team supports custom R&D for formulations like lidocaine patches, ensuring efficacy and safety for chronic conditions.
Contact us today to discuss tailored solutions for your product line.
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- What systemic side effects can lidocaine patches cause? Minimizing Risks for Safe Pain Relief
- For what condition are lidocaine patches approved in the United Kingdom? A Guide to Postherpetic Neuralgia Treatment
- How is the lidocaine patch administered? A Step-by-Step Guide for Safe & Effective Pain Relief
- How are lidocaine patches typically used for pain relief during pregnancy? A Guide to Safe, Targeted Relief
- Are lidocaine patches safe to use during pregnancy? A Guide to Making an Informed Choice













