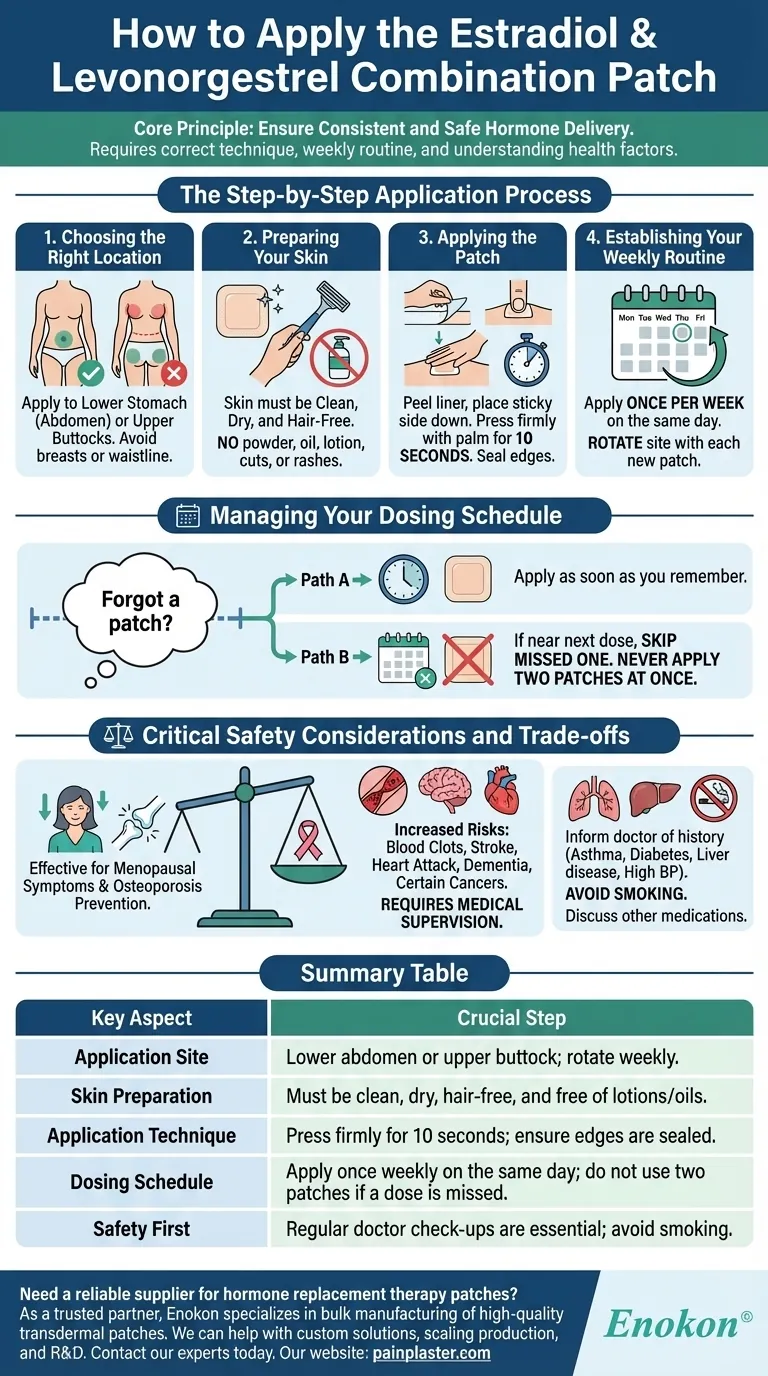
To apply the estradiol and levonorgestrel patch, you must place it on a clean, dry, and hair-free area of your lower stomach or upper buttock. Ensure the skin is free of any powder, oil, or lotion. After peeling off the protective backing, press the patch firmly onto the skin for about 10 seconds to make sure it is sealed correctly.
The core principle of applying this patch is ensuring consistent and safe hormone delivery. This involves not only the physical application technique but also a disciplined weekly routine and an understanding of the critical health factors involved with this therapy.

The Step-by-Step Application Process
Proper application is the foundation for making this medication work as intended. Following a precise method minimizes skin irritation and ensures you receive the correct dose.
Choosing the Right Location
The patch must be applied to the lower stomach (abdomen) or the upper buttocks. These areas provide a good surface for adhesion and consistent absorption.
Avoid applying the patch to your breasts or waistline, where clothing might rub against it and cause it to detach.
Preparing Your Skin
Your skin must be clean, dry, and free of hair. Do not apply any powder, oil, or lotion to the area before application, as this will prevent the patch from sticking properly.
Never apply the patch to skin that is irritated, cut, or has any type of burn or rash.
Applying the Patch
First, carefully peel the protective liner off the patch. Immediately place the sticky side down onto your chosen skin area.
Press down firmly with the palm of your hand for approximately 10 seconds. Run your fingers around the edges to ensure it is sealed completely.
Establishing Your Weekly Routine
This patch is typically applied once per week. It's crucial to change your patch on the same day each week to maintain stable hormone levels.
You must rotate the application site with each new patch. Do not apply a new patch to the exact same spot for at least one week to prevent skin irritation.
Managing Your Dosing Schedule
Consistency is vital for hormone replacement therapy. Knowing how to handle a missed dose prevents disruption to your treatment.
What to Do If You Forget a Patch
If you forget to change your patch on the scheduled day, apply a new one as soon as you remember.
However, if it is almost time for your next scheduled dose, skip the missed one and get back on your regular schedule. Never apply two patches at once to make up for a missed dose.
Critical Safety Considerations and Trade-offs
This medication is effective for treating menopausal symptoms like hot flashes and preventing osteoporosis, but its use involves significant health considerations that require careful medical supervision.
Understanding the Associated Risks
Using this patch can increase the risk of serious health conditions. These include blood clots, stroke, heart attack, dementia, and certain types of cancer, such as breast, endometrial, or uterine cancer. It may also increase the risk of gallbladder disease.
The Importance of Medical Supervision
Regular check-ups with your doctor are essential to monitor for any unwanted effects and to ensure the benefits of the treatment continue to outweigh the risks for you.
Pre-existing Conditions and Lifestyle
Inform your doctor about your complete medical history. Conditions like asthma, diabetes, epilepsy, liver disease, or high blood pressure may be affected by this medication.
It is also critical to avoid smoking, as it significantly increases the risk of serious side effects like blood clots and stroke. Discuss all other medications and supplements you take with your doctor to avoid potential interactions.
Making the Right Choice for Your Goal
- If your primary focus is correct application: Always use a clean, dry, rotated site on the lower abdomen or upper buttocks and press firmly for 10 seconds.
- If your primary focus is managing your treatment schedule: Apply a missed patch as soon as you remember, but never double the dose to make up for it.
- If your primary focus is overall safety: Maintain open and continuous communication with your doctor about all risks, side effects, and any other medications you are taking.
Following these guidelines empowers you to use your medication safely and effectively as part of your overall health plan.
Summary Table:
| Key Aspect | Crucial Step |
|---|---|
| Application Site | Lower abdomen or upper buttock; rotate weekly. |
| Skin Preparation | Must be clean, dry, hair-free, and free of lotions/oils. |
| Application Technique | Press firmly for 10 seconds; ensure edges are sealed. |
| Dosing Schedule | Apply once weekly on the same day; do not use two patches if a dose is missed. |
| Safety First | Regular doctor check-ups are essential; avoid smoking. |
Need a reliable supplier for hormone replacement therapy patches?
As a trusted partner for healthcare distributors and pharmaceutical brands, Enokon specializes in the bulk manufacturing of high-quality, reliable transdermal patches. Our technical expertise ensures consistent drug delivery and superior patient adherence.
We can help you:
- Develop custom transdermal solutions tailored to your active pharmaceutical ingredients (APIs).
- Scale production with rigorous quality control for consistent, batch-to-batch reliability.
- Leverage our R&D expertise to innovate and improve your transdermal product line.
Let's discuss your project. Contact our experts today to explore how our manufacturing capabilities can support your brand's success.
Visual Guide
Related Products
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
People Also Ask
- How are nitroglycerin patches used for angina? A Guide to Safe and Effective Application
- Can transdermal drugs cause skin issues? Understanding Risks & Prevention
- How often should the birth control patch be replaced? A Simple Guide to the Weekly Schedule
- What should you do if someone is exposed to a transdermal patch overdose? Emergency Steps & Prevention
- What is rotigotine transdermal skin patch used to treat? Manage Parkinson's & RLS Symptoms
- How does a high-precision knife coater ensure product quality? Precision Coating for Consistent Drug Delivery
- How is high-precision SEM utilized in characterizing TDDS? Verify Porous Architecture and Nanoparticle Dispersion
- How should one determine the right dose of nicotine patch to use? Match Your Habit for a Successful Quit














