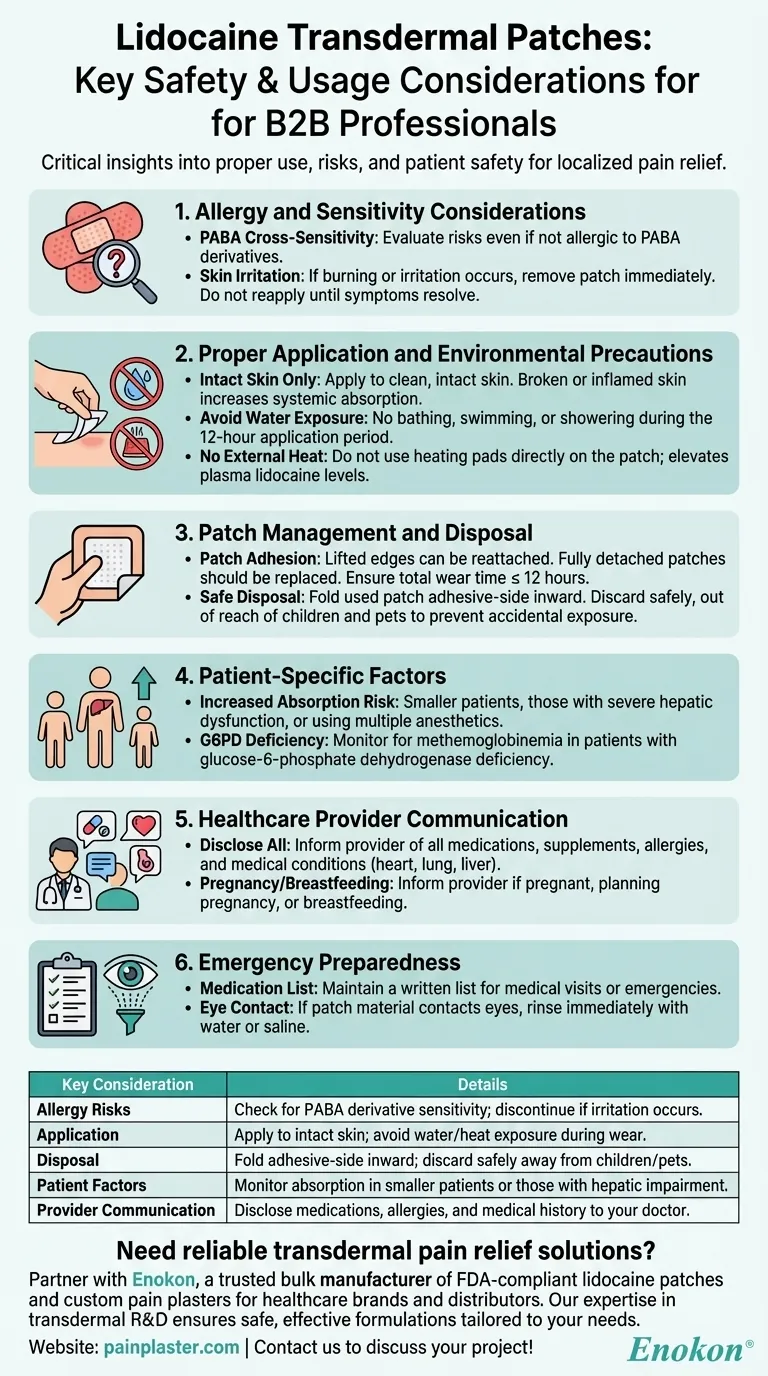
Lidocaine transdermal patches are a common method for localized pain relief, but their use requires careful consideration of several factors to ensure safety and efficacy. Key aspects include potential allergies, proper application techniques, environmental precautions, and monitoring for adverse effects. Patients must be aware of cross-sensitivity risks, especially if allergic to PABA derivatives, and should discontinue use if irritation occurs. Proper disposal is critical to prevent accidental exposure in children or pets. Absorption rates can vary based on skin condition, external heat, and patient-specific factors like hepatic function. Adherence to application guidelines—such as avoiding water exposure and managing patch adhesion—is essential. Patients should also inform healthcare providers about their medical history and current medications to avoid interactions.

Key Points Explained:
-
Allergy and Sensitivity Considerations
- Patients with allergies to para-aminobenzoic acid (PABA) derivatives may not always react to lidocaine, but cross-sensitivity should still be evaluated.
- If skin irritation or burning occurs, the patch should be removed and not reapplied until symptoms resolve.
-
Proper Application and Environmental Precautions
- Apply only to intact skin; broken or inflamed skin can increase systemic absorption.
- Avoid water exposure (e.g., bathing, swimming) during the 12-hour application period.
- Do not apply external heat sources (e.g., heating pads) directly to the patch, as this elevates plasma lidocaine levels.
-
Patch Management and Disposal
- Lifted edges can be reattached, but a fully detached patch should be replaced, ensuring total wear time does not exceed 12 hours.
- After use, fold the patch adhesive-side inward and discard safely out of reach of children and pets.
-
Patient-Specific Factors
- Smaller patients, those with impaired elimination (e.g., severe hepatic dysfunction), or those using multiple anesthetic products may experience higher systemic absorption.
- Monitor for methemoglobinemia in patients with glucose-6-phosphate dehydrogenase deficiency.
-
Healthcare Provider Communication
- Disclose all medications (including supplements), allergies, and medical conditions (e.g., heart, lung, or liver disease) to your doctor.
- Inform providers if pregnant, planning pregnancy, or breastfeeding.
-
Emergency Preparedness
- Maintain a written medication list for medical visits or emergencies.
- If eye contact occurs, rinse immediately with water or saline.
These guidelines highlight how lidocaine transdermal patches blend convenience with nuanced safety protocols—tools that quietly shape modern pain management. Have you considered how these precautions align with your daily routines?
Summary Table:
| Key Consideration | Details |
|---|---|
| Allergy Risks | Check for PABA derivative sensitivity; discontinue if irritation occurs. |
| Application | Apply to intact skin; avoid water/heat exposure during wear. |
| Disposal | Fold adhesive-side inward; discard safely away from children/pets. |
| Patient Factors | Monitor absorption in smaller patients or those with hepatic impairment. |
| Provider Communication | Disclose medications, allergies, and medical history to your doctor. |
Need reliable transdermal pain relief solutions? Partner with Enokon, a trusted bulk manufacturer of FDA-compliant lidocaine patches and custom pain plasters for healthcare brands and distributors. Our expertise in transdermal R&D ensures safe, effective formulations tailored to your needs. Contact us today to discuss your project!
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heat Relief Capsicum Patch for Lower Back Pain Relief
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
People Also Ask
- How should the treated area be protected while wearing a lidocaine patch? Safety Tips for Effective Pain Relief
- How are lidocaine patches typically used for pain relief during pregnancy? A Guide to Safe, Targeted Relief
- Are lidocaine patches safe to use during pregnancy? A Guide to Making an Informed Choice
- When should someone contact a doctor regarding lidocaine patch use? Ensure Safe Pain Relief
- For what condition are lidocaine patches approved in the United Kingdom? A Guide to Postherpetic Neuralgia Treatment
















