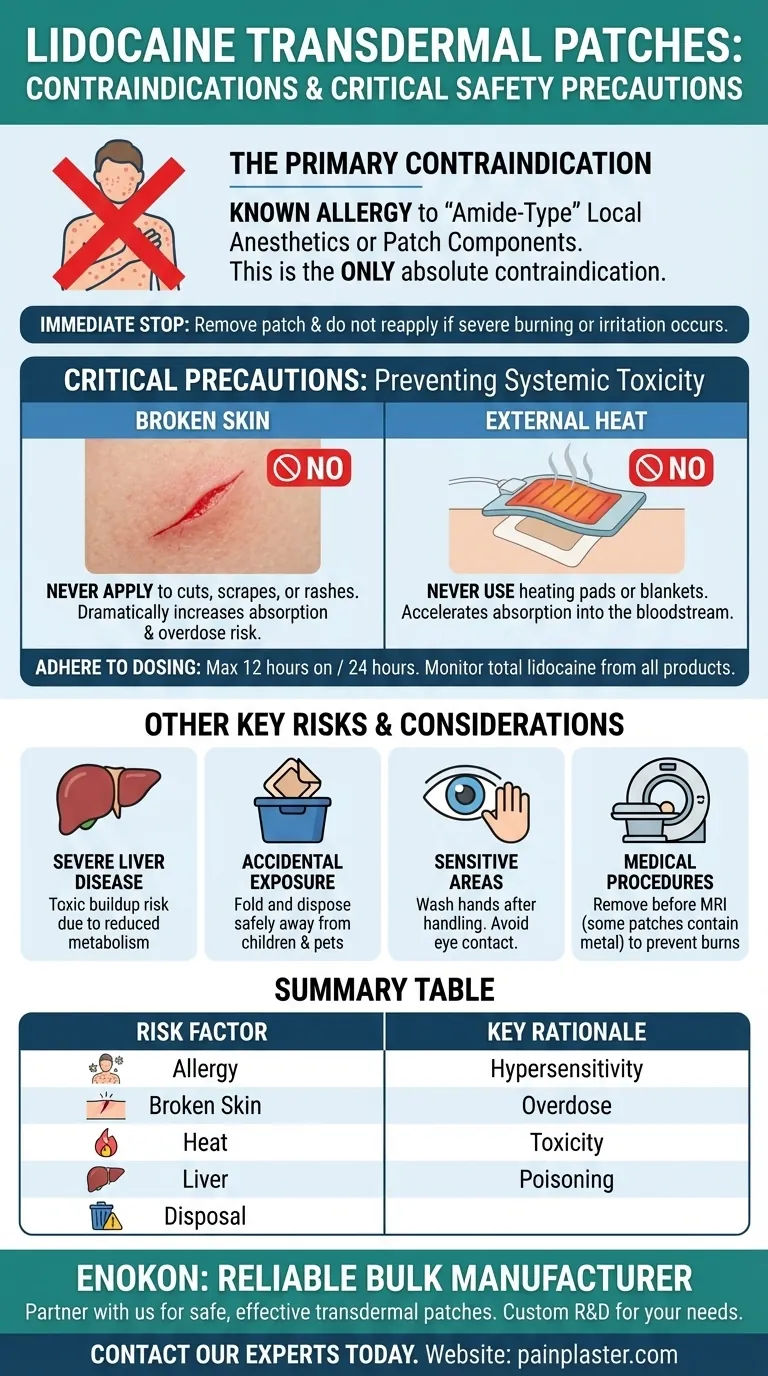
The primary contraindication for a lidocaine transdermal patch is a known history of sensitivity or allergy to local anesthetics of the "amide" type, or to any other component within the patch itself. This is the most critical factor that would prohibit its use.
While a known allergy is the only absolute contraindication, the greatest practical risks come from improper application—specifically, applying patches to broken skin or exposing them to external heat—which can lead to dangerously high levels of lidocaine absorption into your system.
The Primary Contraindication: Known Hypersensitivity
The most important safety check before using a lidocaine patch is confirming the absence of a known allergy. This is non-negotiable.
Understanding Amide-Type Anesthetics
Lidocaine belongs to a class of local anesthetics known as "amides." If you have a documented history of an allergic reaction to lidocaine or similar drugs (like bupivacaine or mepivacaine), you should not use the patch.
Potential Cross-Sensitivity
Patients allergic to older "ester" type anesthetics, which are derived from para-aminobenzoic acid (PABA), do not typically have a cross-sensitivity to amide anesthetics like lidocaine. However, this possibility should still be considered.
Immediate Local Reactions
If you experience significant irritation or a burning sensation after applying the patch, it may indicate a sensitivity. You should remove the patch immediately and not reapply it until the irritation has completely resolved.
Critical Precautions to Prevent Systemic Toxicity
Beyond allergies, the most significant risks involve scenarios that cause your body to absorb too much lidocaine. This is known as systemic toxicity and can have serious consequences.
The Role of Skin Integrity
The patch must only be applied to intact, unbroken skin. Applying it over cuts, scrapes, rashes, or inflamed skin dramatically increases the rate of absorption, raising the risk of overdose.
The Impact of External Heat
Never apply external heat sources, such as heating pads or electric blankets, directly over a lidocaine patch. Heat increases blood flow to the skin, which significantly accelerates the absorption of lidocaine into your bloodstream.
Adherence to Dosing and Duration
It is crucial to follow the prescribed instructions for use. This includes applying only the recommended number of patches and adhering to the specified time limit, which is typically no more than 12 hours of application within a 24-hour period.
Considering Total Lidocaine Exposure
If you are using other products that contain local anesthetic agents (such as gels or creams), the total amount of drug absorbed from all sources must be carefully monitored to prevent cumulative overdose.
Understanding the Trade-offs and Potential Risks
Safe use requires understanding factors that can increase risk and taking steps to mitigate them.
Risk in Patients with Severe Liver Disease
Lidocaine is metabolized by the liver. Patients with severe hepatic impairment may be unable to eliminate the drug effectively, leading to a toxic buildup in the body even with normal use.
Accidental Exposure and Disposal
Used patches still contain a significant amount of lidocaine. After removal, fold the patch in half so the adhesive side sticks to itself. Dispose of it immediately in a location that is safely out of the reach of children and pets to prevent accidental poisoning.
Application Near Sensitive Areas
Wash your hands thoroughly after handling a lidocaine patch. Accidental contact with the eyes can cause severe irritation and requires immediate rinsing with water or saline.
Special Considerations for Medical Procedures
Patches may need to be removed before certain medical procedures. For example, some patches contain metal and must be taken off before an MRI scan to prevent skin burns.
How to Apply This to Your Use
Your approach to using a lidocaine patch should be guided by your primary safety goal.
- If your primary focus is avoiding an allergic reaction: You must confirm you have no known sensitivity to amide-type local anesthetics before the first use.
- If your primary focus is preventing accidental overdose: Never apply the patch to broken skin, strictly limit application time to 12 hours, and never use external heat sources over the patch.
- If your primary focus is protecting others: Always wash your hands after handling a patch and ensure used patches are folded and disposed of where children or pets cannot access them.
Understanding these principles is the key to using a lidocaine patch both safely and effectively.
Summary Table:
| Contraindication / Precaution | Key Risk / Rationale |
|---|---|
| Known Allergy to Amide Anesthetics | Hypersensitivity reaction (e.g., to lidocaine, bupivacaine). |
| Application to Broken Skin | Dramatically increases absorption, risk of overdose. |
| Use with External Heat | Accelerates absorption, leading to systemic toxicity. |
| Severe Liver Impairment | Reduced metabolism can cause toxic drug buildup. |
| Improper Disposal | Risk of accidental poisoning of children or pets. |
Need a reliable, safe transdermal patch solution?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we partner with healthcare and pharma distributors and brands to ensure product safety and efficacy. Our technical expertise supports custom R&D to meet your specific requirements, helping you deliver trusted products to the market.
Contact our experts today to discuss your transdermal patch needs.
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
People Also Ask
- When should someone contact a doctor regarding lidocaine patch use? Ensure Safe Pain Relief
- For what condition are lidocaine patches approved in the United Kingdom? A Guide to Postherpetic Neuralgia Treatment
- How is the lidocaine patch administered? A Step-by-Step Guide for Safe & Effective Pain Relief
- Are lidocaine patches safe to use during pregnancy? A Guide to Making an Informed Choice
- How should the treated area be protected while wearing a lidocaine patch? Safety Tips for Effective Pain Relief















