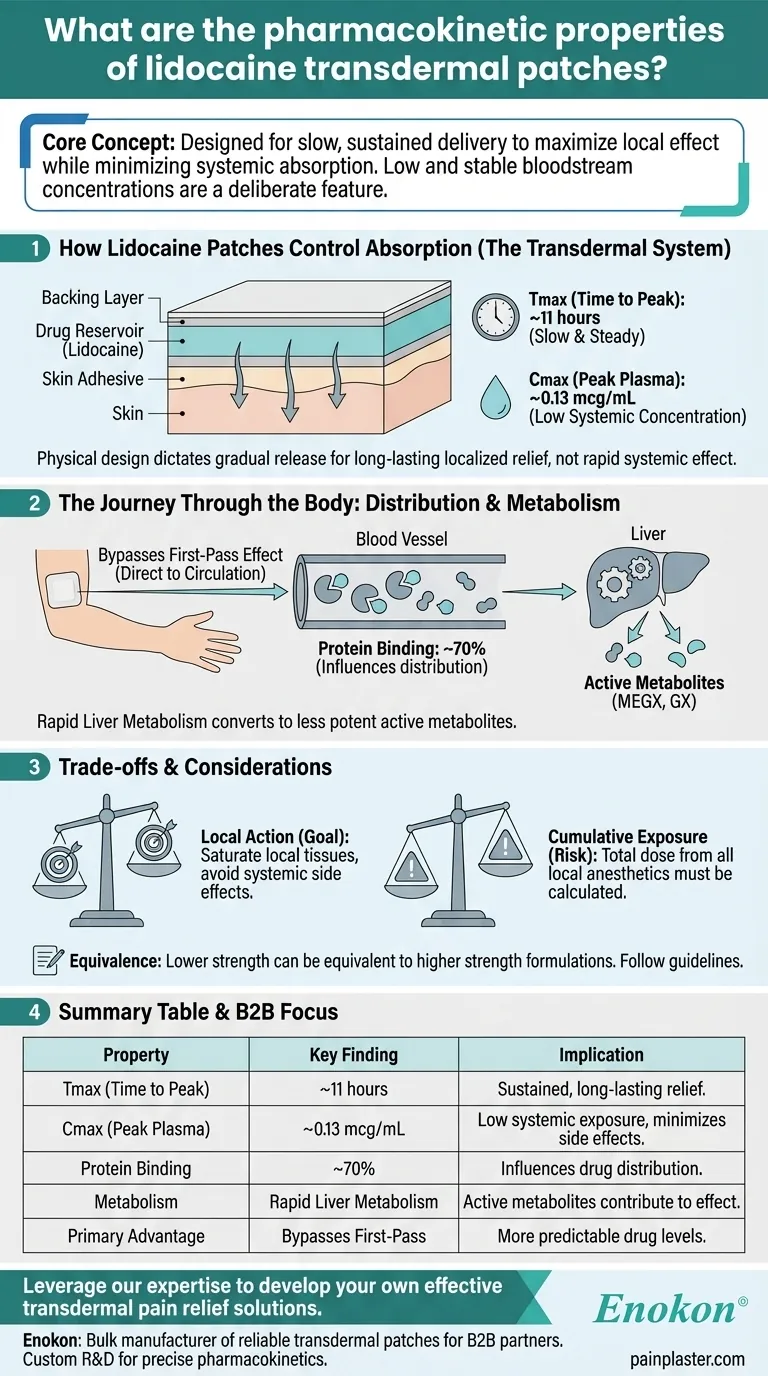
At its core, the lidocaine transdermal patch is designed for slow, sustained drug delivery, resulting in low and stable concentrations in the bloodstream. Its pharmacokinetic profile is characterized by a long time to reach peak plasma concentration (approximately 11 hours), significant protein binding (70%), and rapid metabolism by the liver into less potent active metabolites.
The key takeaway is that lidocaine patches are engineered to maximize local anesthetic effects while minimizing systemic absorption and toxicity. Their unique pharmacokinetics—slow absorption and low peak plasma levels—are a deliberate feature, not a limitation.
How Lidocaine Patches Control Absorption
The physical design of a transdermal patch is what dictates its pharmacokinetic behavior. It's a system built for controlled, gradual release directly through the skin.
The Transdermal Delivery System
A lidocaine patch consists of multiple layers, including a backing layer, a drug reservoir containing the lidocaine, and a skin adhesive. The medication slowly moves from the reservoir, through the adhesive, and is absorbed through the skin into the local tissues.
Slow and Steady Release
This design results in a very slow rate of absorption. The time to reach peak plasma concentration (Tmax) is approximately 11 hours. This slow onset is fundamental to its purpose of providing long-lasting, localized relief rather than a rapid systemic effect.
Low Systemic Concentration
Even when using multiple patches, the peak plasma concentration (Cmax) remains very low—around 0.13 mcg/mL with three patches applied. This confirms the patch's primary action is at the application site, not throughout the body.
The Journey Through the Body: Distribution and Metabolism
Once the small amount of lidocaine is absorbed into the bloodstream, it follows a predictable path of distribution and breakdown.
Bypassing the First-Pass Effect
A major advantage of the transdermal route is that it bypasses the "first-pass effect." Unlike oral medications that go directly to the liver after absorption, the patch delivers the drug into circulation first, leading to more predictable drug levels.
Protein Binding and Distribution
Once in the blood, about 70% of lidocaine binds to plasma proteins. Its volume of distribution (Vd) is between 0.7 and 2.7 L/kg, which describes how it distributes from the blood into the body's tissues.
Rapid Liver Metabolism
Lidocaine that enters the systemic circulation is metabolized rapidly by the liver. It is converted into active metabolites, primarily monoethylglycinexylidide (MEGX) and glycinexylidide (GX), which have similar but less potent anesthetic properties.
Understanding the Trade-offs and Considerations
The unique pharmacokinetic profile of the lidocaine patch creates distinct advantages but also requires specific clinical considerations.
Local Action is the Goal
The slow absorption and low systemic concentration are intentional design features. They allow the drug to saturate local tissues for pain relief while keeping blood levels far below what would be needed for systemic anesthetic or antiarrhythmic effects, thus avoiding associated side effects.
Cumulative Exposure is a Risk
It is critical to remember that the absorbed dose is cumulative. If a patient is using a lidocaine patch along with other products containing local anesthetics, the total amount of drug absorbed from all formulations must be calculated to prevent systemic toxicity.
Equivalence Between Strengths
Lidocaine patches come in various strengths, such as 1.8% and 5%. It's important to note that due to differences in formulation, a lower strength patch can provide equivalent drug exposure to a higher strength one. Always follow specific product guidelines.
Making the Right Choice for Your Goal
Understanding these pharmacokinetic properties allows for the safe and effective use of the lidocaine patch.
- If your primary focus is rapid pain relief: The lidocaine patch is not the ideal choice due to its very slow time to peak effect (around 11 hours).
- If your primary focus is sustained, localized relief with minimal systemic side effects: The patch is an excellent option, as its pharmacokinetic profile is specifically engineered for low, steady systemic absorption.
- If your patient is using multiple anesthetic products: You must carefully consider the total cumulative dose from all sources to prevent systemic toxicity, as the patch contributes to the total body burden of the drug.
By aligning the tool to the clinical task, you leverage the distinct pharmacokinetic profile of the lidocaine patch for optimal patient outcomes.
Summary Table:
| Property | Key Finding | Implication |
|---|---|---|
| Tmax (Time to Peak) | ~11 hours | Provides sustained, long-lasting relief, not rapid onset. |
| Cmax (Peak Plasma Concentration) | ~0.13 mcg/mL (with 3 patches) | Ensures low systemic exposure, minimizing side effects. |
| Protein Binding | ~70% | Influences drug distribution and availability. |
| Metabolism | Rapidly metabolized by the liver | Active metabolites contribute to the therapeutic effect. |
| Primary Advantage | Bypasses first-pass metabolism | Leads to more predictable drug levels in the body. |
Leverage our expertise to develop your own effective transdermal pain relief solutions.
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters. We partner with healthcare and pharmaceutical distributors and brands to bring their products to market.
Our technical expertise in custom R&D and formulation development ensures your patches deliver the precise pharmacokinetic profile required for safe and effective patient outcomes.
Contact our team today to discuss your custom transdermal patch development needs.
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- How can you use lidocaine patches for multiple sore spots? A Guide to Safe, Effective Pain Relief
- For what condition are lidocaine patches approved in the United Kingdom? A Guide to Postherpetic Neuralgia Treatment
- How should the treated area be protected while wearing a lidocaine patch? Safety Tips for Effective Pain Relief
- Is it safe to use lidocaine patches while breastfeeding? Expert Guidance for Nursing Mothers
- How does the lidocaine patch work? Targeted Relief for Nerve Pain Explained














