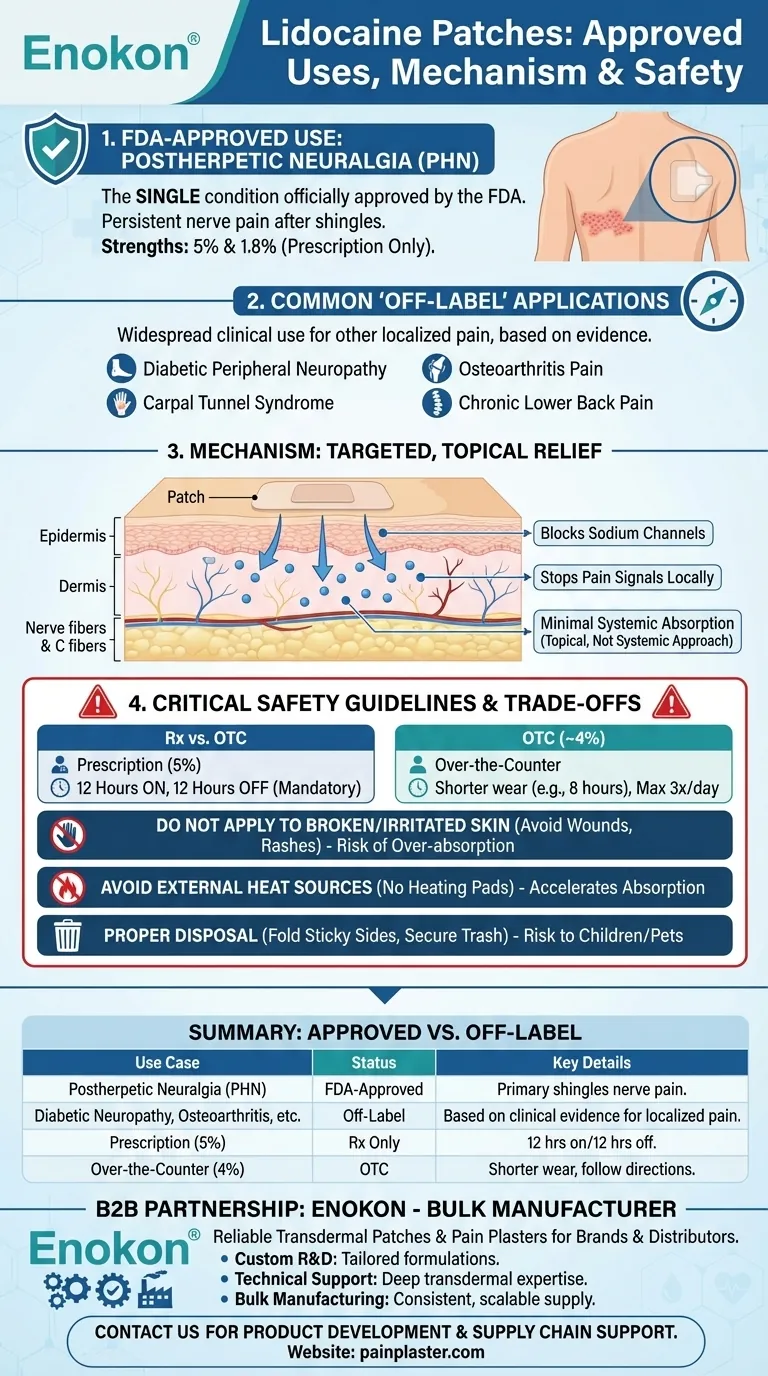
Lidocaine patches are officially approved by the U.S. Food and Drug Administration (FDA) for a single medical condition. That condition is postherpetic neuralgia (PHN), the persistent and often debilitating nerve pain that can linger after a shingles infection has resolved. Both 5% and 1.8% strength prescription patches are specifically indicated for this use.
While the patch's formal approval is narrow, its mechanism of delivering targeted, localized pain relief has led to its widespread "off-label" use for other pain conditions. The key is understanding that its primary function is to numb the nerves directly beneath the skin where it is applied.

The Approved Use vs. Common Practice
To use lidocaine patches effectively and safely, it's crucial to distinguish between their official indication and how they are often used in clinical practice.
The Official FDA Indication: Postherpetic Neuralgia (PHN)
Postherpetic neuralgia is a complication of the shingles virus. It damages nerve fibers, causing burning, shooting, or aching pain that can last for months or even years after the initial rash disappears.
The lidocaine patch is considered a first-line treatment for PHN because it delivers the anesthetic directly to the painful area with minimal absorption into the rest of the body.
Common "Off-Label" Applications
Physicians often prescribe medications for uses that are not officially approved by the FDA, a practice known as "off-label" use. This is common when there is scientific evidence suggesting a drug is effective for other conditions.
Research and clinical experience suggest lidocaine patches may be effective for other localized pain conditions, including:
- Diabetic peripheral neuropathy
- Carpal tunnel syndrome
- Osteoarthritis pain
- Chronic lower back pain
How Lidocaine Patches Provide Targeted Relief
The patch's effectiveness comes from its simple and direct mechanism of action.
Blocking Local Pain Signals
Lidocaine is a local anesthetic. When applied via a patch, it seeps into the skin and blocks sodium channels within the nerve endings.
These specific nerve fibers, known as A-delta and C fibers, are responsible for transmitting pain signals. By blocking them, the patch essentially stops the pain message from ever leaving the affected area and reaching the brain.
A Topical, Not Systemic, Approach
Unlike oral pain medications that circulate throughout your entire body, a lidocaine patch provides relief only where you apply it. This significantly reduces the risk of systemic side effects.
Critical Safety Guidelines and Trade-offs
Proper use is essential to ensure both safety and effectiveness. The rules are not suggestions; they are necessary precautions.
Prescription vs. Over-the-Counter (OTC)
There is a key difference in strength and usage instructions. Prescription patches (5%) are typically worn for up to 12 hours, followed by a mandatory 12-hour "off" period.
Over-the-counter patches (usually 4%) have a shorter wear time, often up to 8 hours, and should not be used more than three times a day.
Do Not Apply to Broken or Irritated Skin
This is the most important rule. The skin must be clean, dry, and fully intact. Applying a patch to open wounds, rashes, or inflamed skin can cause the body to absorb too much lidocaine, increasing the risk of adverse effects.
Avoid External Heat Sources
Never use a heating pad, electric blanket, or other heat source over a lidocaine patch. Heat increases blood flow to the skin, which can accelerate lidocaine absorption to potentially dangerous levels.
Proper Application and Disposal
For best results, trim any excess hair (do not shave) and apply the patch to a flat area where it won't be rubbed by tight clothing.
Used patches still contain a significant amount of medication. They pose a serious risk to children and pets if ingested. Always dispose of them by folding the sticky sides together and placing them in a trash can out of reach.
Making the Right Choice for Your Pain
Your specific type of pain and its location will determine if a lidocaine patch is a suitable option.
- If your primary focus is diagnosed postherpetic neuralgia (shingles pain): A prescription lidocaine patch is an FDA-approved, standard treatment that you should discuss with your healthcare provider.
- If your primary focus is other localized nerve, joint, or muscle pain: While not officially approved for these uses, a lidocaine patch may offer targeted relief, but it is essential to consult a professional to confirm it's appropriate for your specific condition.
- If your primary focus is trying an over-the-counter option: You must follow the package directions precisely, paying close attention to the maximum wear time and never applying it to damaged skin.
Understanding both the approved uses and the critical safety rules empowers you to make an informed decision about managing your localized pain.
Summary Table:
| Use Case | Status | Key Details |
|---|---|---|
| Postherpetic Neuralgia (PHN) | FDA-Approved | Primary, official indication for shingles-related nerve pain. |
| Diabetic Neuropathy, Osteoarthritis, etc. | Off-Label | Commonly prescribed based on clinical evidence for localized pain. |
| Prescription (5%) | Rx Only | Wear up to 12 hours, then 12 hours off. |
| Over-the-Counter (4%) | OTC | Shorter wear time (e.g., 8 hours); follow package directions. |
Need a reliable, high-quality lidocaine patch for your brand or distribution network?
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters. We partner with healthcare and pharmaceutical distributors and brands to deliver effective topical pain relief solutions.
Benefit from our expertise:
- Custom R&D: We can tailor formulations to meet your specific market needs.
- Technical Support: Leverage our deep knowledge of transdermal delivery systems.
- Bulk Manufacturing: Scale your supply with consistent, high-quality production.
Contact our team today to discuss how we can support your product development and supply chain.
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- For what condition are lidocaine patches approved in the United Kingdom? A Guide to Postherpetic Neuralgia Treatment
- Is it safe to use lidocaine patches while breastfeeding? Expert Guidance for Nursing Mothers
- How can you use lidocaine patches for multiple sore spots? A Guide to Safe, Effective Pain Relief
- Are lidocaine patches safe to use during pregnancy? A Guide to Making an Informed Choice
- How should the treated area be protected while wearing a lidocaine patch? Safety Tips for Effective Pain Relief
















