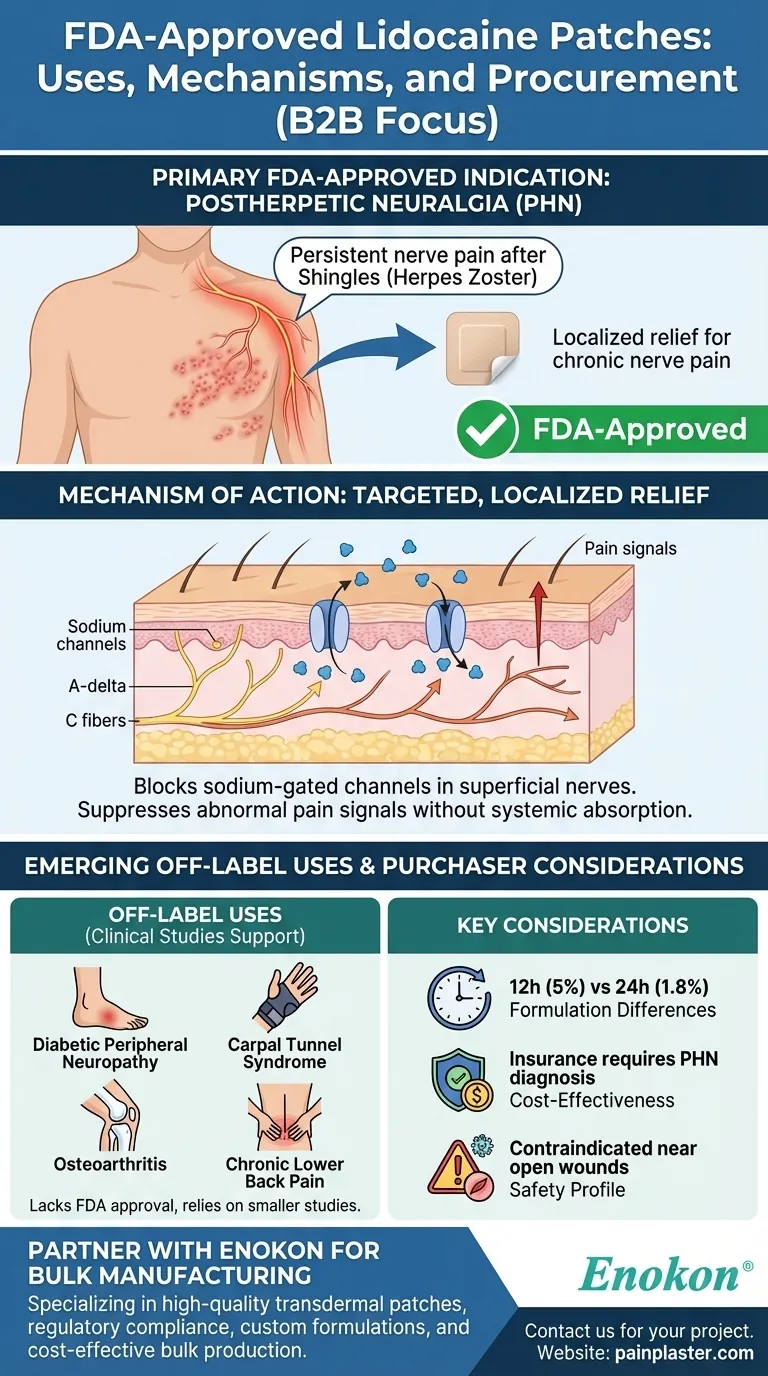
Prescription lidocaine patches are FDA-approved for specific conditions, primarily postherpetic neuralgia (PHN), a persistent nerve pain complication following shingles. While research suggests potential off-label uses for other neuropathic and musculoskeletal pain conditions, the FDA's formal approval remains limited to PHN. These patches provide localized relief by blocking sodium channels in superficial nerve fibers, making them a targeted option for certain types of chronic pain.

Key Points Explained:
-
Primary FDA-Approved Indication: Postherpetic Neuralgia (PHN)
- The FDA has approved both 5% and 1.8% prescription lidocaine patches exclusively for PHN, a chronic pain condition caused by nerve damage after shingles (herpes zoster infection).
- PHN manifests as burning, stabbing, or hypersensitivity in areas previously affected by the shingles rash, often lasting months to years.
-
Mechanism of Action
- Lidocaine patches work by blocking sodium-gated channels in localized A-delta and C nerve fibers just beneath the skin.
- This action suppresses abnormal pain signals without systemic absorption, minimizing side effects compared to oral medications.
-
Off-Label Uses Supported by Research While not FDA-approved, clinical studies suggest efficacy for:
- Diabetic Peripheral Neuropathy: Pain from nerve damage due to diabetes.
- Carpal Tunnel Syndrome: Compression neuropathy of the median nerve.
- Osteoarthritis: Joint pain, particularly in superficial areas like the knees.
- Chronic Lower Back Pain: Especially when neuropathic components are present.
-
Considerations for Purchasers
- Formulation Differences: 5% patches (e.g., Lidoderm) are applied for 12 hours/day, while 1.8% patches (e.g., ZTlido) allow for 24-hour wear due to enhanced adhesion.
- Cost-Effectiveness: Insurance coverage typically requires PHN diagnosis; off-label use may necessitate prior authorization.
- Safety Profile: Contraindicated near open wounds or infections; minimal drug interactions make them suitable for elderly patients.
-
Regulatory Context
- The FDA's approval is based on rigorous PHN-specific trials demonstrating statistically significant pain reduction.
- Off-label applications rely on smaller studies or clinician experience, lacking the same level of regulatory scrutiny.
For healthcare purchasers, understanding these distinctions ensures appropriate procurement and formulary decisions, balancing approved indications with emerging evidence for other pain syndromes.
Summary Table:
| Key Aspect | Details |
|---|---|
| FDA-Approved Use | Postherpetic neuralgia (PHN) – chronic nerve pain after shingles. |
| Mechanism of Action | Blocks sodium channels in superficial nerves for localized pain relief. |
| Off-Label Uses | Diabetic neuropathy, carpal tunnel syndrome, osteoarthritis, chronic back pain. |
| Formulation Options | 5% (12-hour wear) or 1.8% (24-hour wear) patches. |
| Safety & Coverage | Minimal systemic absorption; insurance often requires PHN diagnosis. |
Need reliable, FDA-compliant lidocaine patches for your healthcare facility or brand?
At Enokon, we specialize in bulk manufacturing of high-quality transdermal patches, including prescription-grade pain relief solutions. Our expertise ensures:
- Regulatory compliance for PHN treatment and other pain management needs
- Custom formulations to match your specific requirements
- Cost-effective bulk production for distributors and healthcare brands
Contact our team today to discuss your lidocaine patch procurement or custom development project.
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- What systemic side effects can lidocaine patches cause? Minimizing Risks for Safe Pain Relief
- When should someone contact a doctor regarding lidocaine patch use? Ensure Safe Pain Relief
- For what condition are lidocaine patches approved in the United Kingdom? A Guide to Postherpetic Neuralgia Treatment
- Are lidocaine patches safe to use during pregnancy? A Guide to Making an Informed Choice
- How are lidocaine patches typically used for pain relief during pregnancy? A Guide to Safe, Targeted Relief














