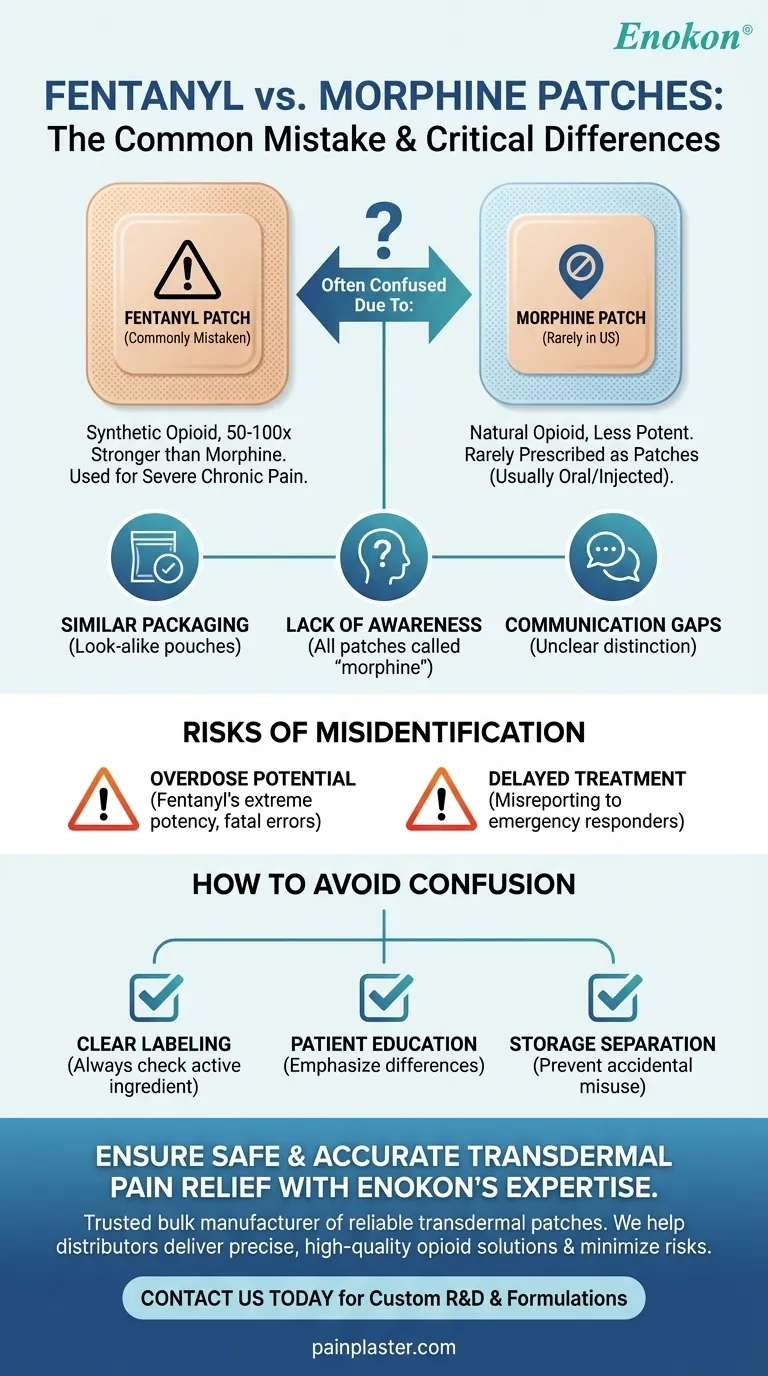
Fentanyl patches are commonly mistaken for morphine patches in the United States. Both are opioid-based pain relief medications, but they differ in active ingredients, potency, and application. Fentanyl patches are transdermal systems used for chronic severe pain, while morphine patches are less commonly prescribed and often confused due to similar packaging or patient misunderstanding. This mix-up can lead to serious health risks, as fentanyl is significantly more potent than morphine.

Key Points Explained:
-
Fentanyl vs. Morphine Patches
- Fentanyl Patches: These are transdermal patches containing fentanyl, a synthetic opioid 50-100 times stronger than morphine. They are prescribed for severe, chronic pain, often in cancer patients.
- Morphine Patches: While morphine is a well-known opioid, transdermal morphine patches are rare in the U.S. Most morphine is administered orally or via injection. Confusion arises because patients or caregivers may assume any opioid patch is a "morphine patch."
-
Reasons for Confusion
- Similar Packaging: Both types of patches may come in small, adhesive pouches, leading to mix-ups.
- Lack of Awareness: Patients unfamiliar with opioid classifications might refer to any pain patch as a "morphine patch."
- Healthcare Communication Gaps: Pharmacists or prescribers may not always clarify the distinction, especially if patients have prior experience with morphine.
-
Risks of Misidentification
- Overdose Potential: Fentanyl's extreme potency means even small dosage errors can be fatal. Mistaking it for morphine could lead to accidental overdose.
- Delayed Treatment: If a patient reports using a "morphine patch" but is actually on fentanyl, medical responders might underestimate the opioid involved.
-
How to Avoid Confusion
- Clear Labeling: Always check the prescription label for the active ingredient (e.g., morphine pain patch vs. fentanyl).
- Patient Education: Healthcare providers should emphasize the differences during prescribing.
- Storage Separation: Keep opioid patches separate from other medications to prevent accidental misuse.
-
Regulatory and Safety Measures
- The FDA and DEA monitor fentanyl patches closely due to their high abuse potential. Pharmacies often require additional verification for these prescriptions.
Understanding these distinctions is critical for safe use, especially given the opioid crisis. Have you considered how such mix-ups might affect emergency medical responses? This underscores the importance of precise medication labeling and patient awareness in everyday healthcare.
Summary Table:
| Aspect | Fentanyl Patches | Morphine Patches |
|---|---|---|
| Active Ingredient | Fentanyl (50-100x stronger than morphine) | Morphine (rarely in patch form) |
| Common Use | Severe chronic pain (e.g., cancer patients) | Rarely prescribed as patches; usually oral/injected |
| Risk of Confusion | High due to similar packaging and patient misunderstanding | Often mislabeled by patients unfamiliar with opioids |
| Potential Consequences | Accidental overdose (fentanyl is extremely potent) | Delayed treatment if misreported in emergencies |
Ensure safe and accurate transdermal pain relief with Enokon’s expertise.
As a trusted bulk manufacturer of reliable transdermal patches and pain plasters, we help healthcare distributors and brands deliver precise, high-quality opioid solutions. Our technical team supports custom R&D to minimize risks like mislabeling or dosage errors.
Contact us today to discuss your needs and benefit from our industry-leading formulations.
Visual Guide
Related Products
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
- Heating Pain Relief Patches for Menstrual Cramps
People Also Ask
- Can the pain relief patch be used with other external analgesic products? A Critical Safety Guide
- Can pregnant women use pain relief patches? Your Essential Guide to Safe Pain Management
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing
- Can children use the pain relief patch? A Critical Safety Guide for Parents
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
















