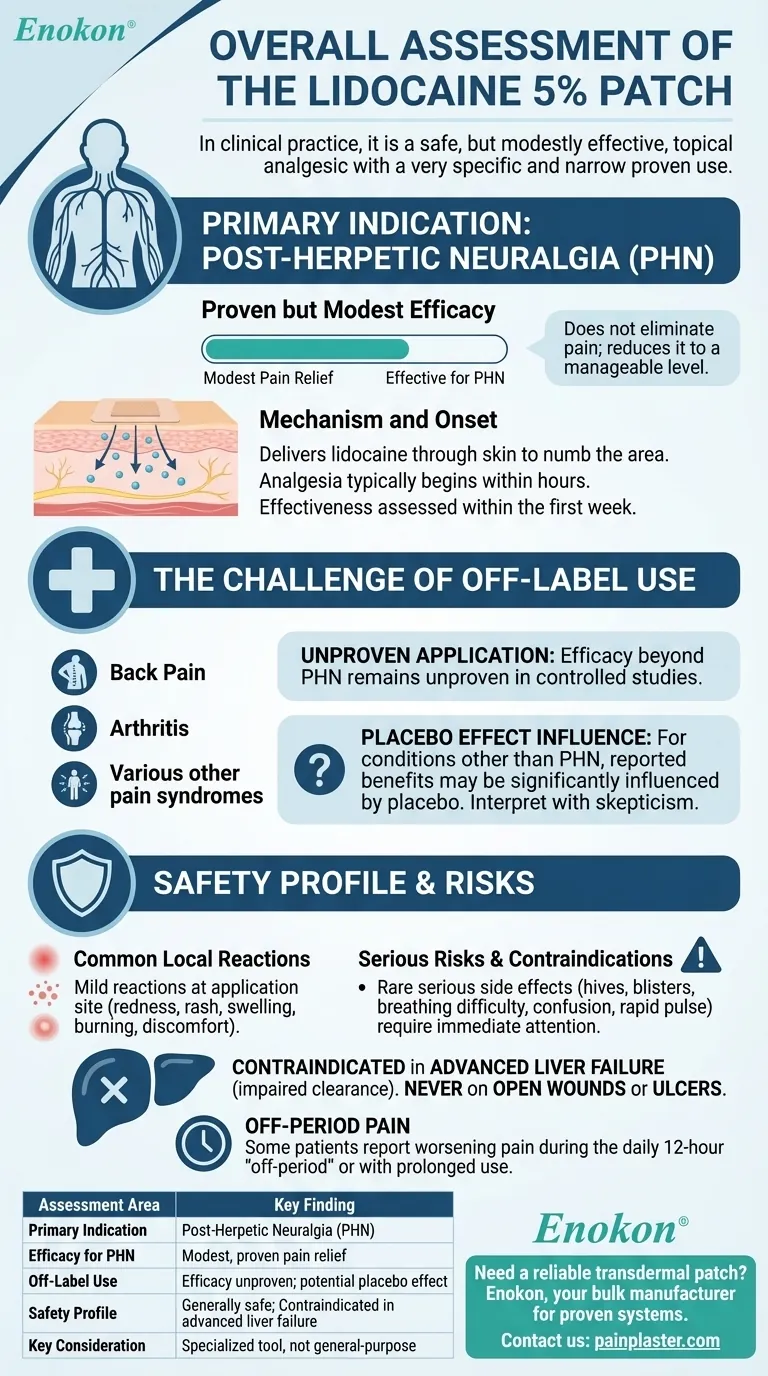
In clinical practice, the overall assessment of the lidocaine 5% patch is that of a safe, but only modestly effective, topical analgesic with a very specific and narrow field of proven use. Its primary, evidence-backed indication is for pain associated with post-herpetic neuralgia (PHN). For the wide variety of other pain syndromes where it is used off-label, its effectiveness is unproven and any reported benefits may be largely due to a placebo effect.
The lidocaine patch is best understood as a specialized tool for post-herpetic neuralgia, offering a high safety profile but modest pain relief. For all other pain conditions, its use is considered off-label, and any perceived benefits should be viewed with significant clinical caution.

The Core Indication: Post-Herpetic Neuralgia
Proven but Modest Efficacy
The lidocaine patch is an established treatment for post-herpetic neuralgia, the persistent nerve pain that can follow a shingles outbreak.
While it is effective for this condition, the degree of pain relief is consistently described as modest. It does not eliminate pain but can reduce it to a more manageable level for some patients.
Mechanism and Onset
The patch delivers lidocaine, a local anesthetic, directly through the skin to numb the painful area.
Analgesia typically begins within a few hours of application. A clinician can generally assess the patch's effectiveness for a patient within the first week of use.
The Challenge of Widespread Off-Label Use
An Unproven Application
Despite its specific indication, the lidocaine patch is widely used for various other pain syndromes, from back pain to arthritis.
This widespread use is not supported by robust clinical evidence. The efficacy beyond PHN remains unproven in controlled studies.
The Influence of Placebo
For pain conditions other than PHN, reported benefits from the lidocaine patch may be significantly influenced by the placebo effect.
Clinicians are advised to interpret non-controlled reports and anecdotal claims of efficacy with a high degree of skepticism.
Understanding the Trade-offs and Safety Profile
Common Local Reactions
The most frequent adverse reactions occur at the application site and are typically mild.
These can include redness, rash, swelling, burning, or general discomfort where the patch was applied.
Serious Risks and Contraindications
Serious side effects are rare but require immediate medical attention. These include hives, blisters, difficulty breathing, confusion, or a rapid pulse.
The patch is contraindicated in patients with advanced liver failure, as their ability to clear lidocaine from the system is impaired. It should also never be used on open wounds or ulcers due to a lack of safety data.
The "Off-Period" Pain
Some patients report that their pain worsens during the daily 12-hour "off-period" when the patch is removed.
Similarly, prolonged use beyond the recommended time can sometimes lead to an increase in pain.
How to Apply This to Your Goal
To use this information effectively, align your strategy with the specific clinical scenario.
- If your primary focus is treating diagnosed Post-Herpetic Neuralgia (PHN): The lidocaine patch is a safe and reasonable option to consider for modest, localized pain relief.
- If your primary focus is treating other types of chronic pain: Proceed with caution, as the patch's efficacy is unproven for these conditions and any benefit may simply be a placebo effect.
- If patient safety is the absolute priority: The patch is generally very safe for topical use, but you must screen for advanced liver failure and avoid application on broken skin.
Ultimately, understanding the lidocaine patch's specific indication and limitations is the key to responsible and effective application.
Summary Table:
| Assessment Area | Key Finding |
|---|---|
| Primary Indication | Post-Herpetic Neuralgia (PHN) |
| Efficacy for PHN | Modest, proven pain relief |
| Off-Label Use | Efficacy unproven; potential placebo effect |
| Safety Profile | Generally safe with mild local reactions; contraindicated in advanced liver failure |
| Key Consideration | A specialized tool, not a general-purpose analgesic |
Need a reliable transdermal patch for your analgesic product line?
As a bulk manufacturer of proven transdermal delivery systems, Enokon partners with healthcare and pharmaceutical brands to develop effective pain management solutions. Our technical expertise in custom R&D ensures your product is backed by robust formulation and manufacturing excellence.
Let's discuss your project: Contact our experts today to explore how we can support your brand's success.
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- For what condition are lidocaine patches approved in the United Kingdom? A Guide to Postherpetic Neuralgia Treatment
- What systemic side effects can lidocaine patches cause? Minimizing Risks for Safe Pain Relief
- How is the lidocaine patch administered? A Step-by-Step Guide for Safe & Effective Pain Relief
- How does the lidocaine patch work? Targeted Relief for Nerve Pain Explained
- How should the treated area be protected while wearing a lidocaine patch? Safety Tips for Effective Pain Relief















