The effectiveness of the lidocaine patch 5 percent was assessed using a combination of standardized pain scales, patient/investigator-reported outcomes, and quality-of-life metrics. These measures were designed to capture both quantitative and qualitative improvements in pain management, functionality, and overall satisfaction. The study followed an open-label, non-randomized design across multiple U.S. sites, with patients applying up to four patches daily while maintaining existing analgesic regimens.
Key Points Explained:
-
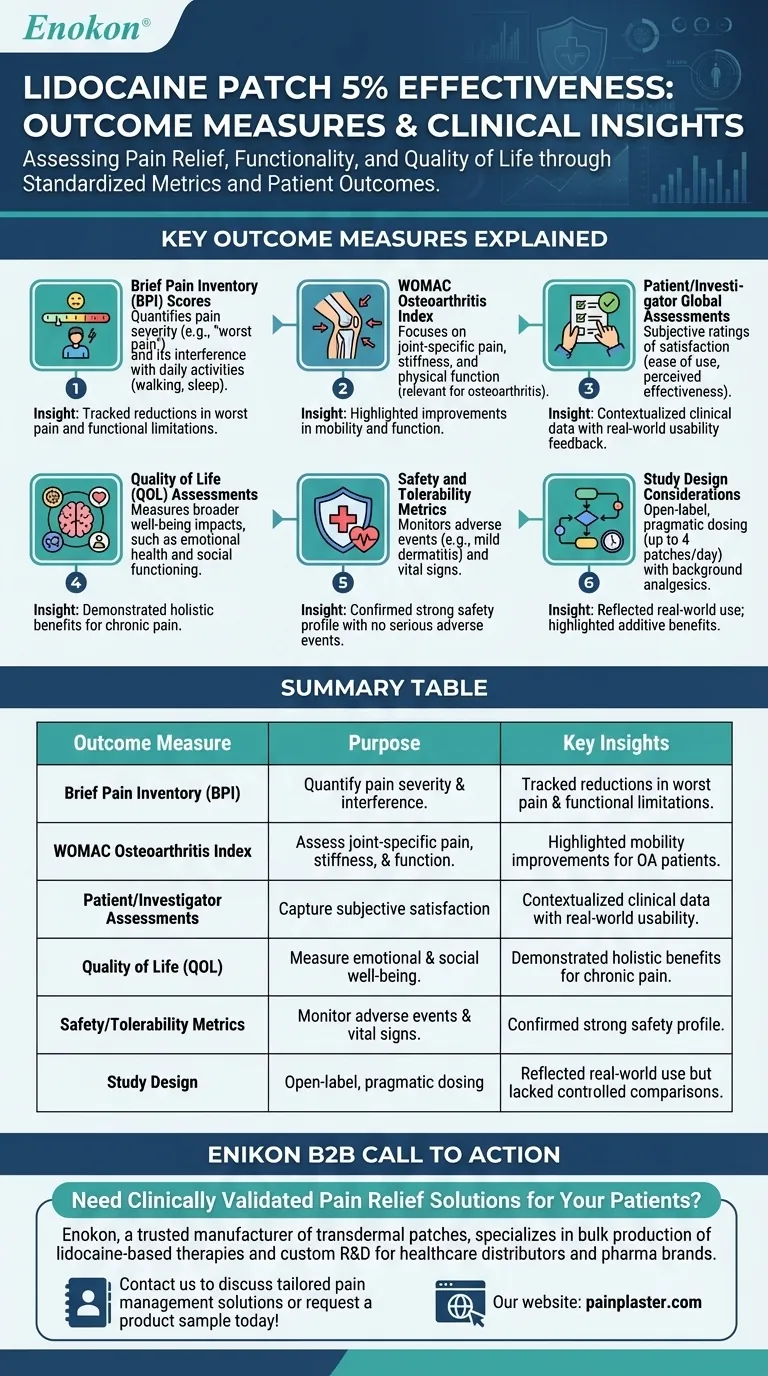
Brief Pain Inventory (BPI) Scores
- A validated tool to evaluate pain severity (e.g., "worst pain in the last 24 hours") and its impact on daily activities (e.g., walking, sleep).
- Used to quantify changes in pain intensity and interference after patch application.
-
WOMAC Osteoarthritis Index
- Focused on joint-specific pain, stiffness, and physical function, particularly relevant for osteoarthritis-related pain.
- Provided insights into functional improvements beyond general pain relief.
-
Patient/Investigator Global Assessments
- Subjective ratings of patch satisfaction (e.g., ease of use, perceived effectiveness).
- Helped contextualize clinical data with real-world usability feedback.
-
Quality of Life (QOL) Assessments
- Measured broader well-being impacts, such as emotional health and social functioning.
- Critical for evaluating holistic benefits in chronic pain conditions like PHN or diabetic neuropathy.
-
Safety and Tolerability Metrics
- Monitored adverse events (e.g., mild dermatitis, headaches) and vital signs.
- No serious adverse events or drug interactions were reported, supporting its safety profile.
-
Study Design Considerations
- Open-label, non-randomized design may introduce bias, but pragmatic dosing (up to 4 patches/day) reflected real-world use.
- Combined with stable background analgesics, outcomes highlighted additive benefits rather than standalone efficacy.
These measures collectively addressed pain reduction, functional gains, and patient-centric outcomes, though the lack of controlled data warrants cautious interpretation for off-label uses.
Summary Table:
| Outcome Measure | Purpose | Key Insights |
|---|---|---|
| Brief Pain Inventory (BPI) | Quantify pain severity and interference with daily activities. | Tracked reductions in worst pain and functional limitations. |
| WOMAC Osteoarthritis Index | Assess joint-specific pain, stiffness, and physical function. | Highlighted improvements in mobility for osteoarthritis patients. |
| Patient/Investigator Assessments | Capture subjective satisfaction (ease of use, perceived relief). | Contextualized clinical data with real-world usability feedback. |
| Quality of Life (QOL) | Measure emotional health and social functioning impacts. | Demonstrated holistic benefits for chronic pain conditions. |
| Safety/Tolerability Metrics | Monitor adverse events (e.g., dermatitis) and vital signs. | Confirmed strong safety profile with no serious adverse events. |
| Study Design | Open-label, pragmatic dosing (up to 4 patches/day) with background analgesics. | Reflected real-world use but lacked controlled comparisons. |
Need clinically validated pain relief solutions for your patients?
As a trusted manufacturer of transdermal patches, Enokon specializes in bulk production of lidocaine-based therapies and custom R&D for healthcare distributors and pharma brands. Our expertise ensures reliable, patient-centric formulations backed by rigorous outcome measures.
Contact us to discuss tailored pain management solutions or request a product sample today!
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How can you use lidocaine patches for multiple sore spots? A Guide to Safe, Effective Pain Relief
- How does the lidocaine patch work? Targeted Relief for Nerve Pain Explained
- How are lidocaine patches typically used for pain relief during pregnancy? A Guide to Safe, Targeted Relief
- Are lidocaine patches safe to use during pregnancy? A Guide to Making an Informed Choice
- When should someone contact a doctor regarding lidocaine patch use? Ensure Safe Pain Relief














