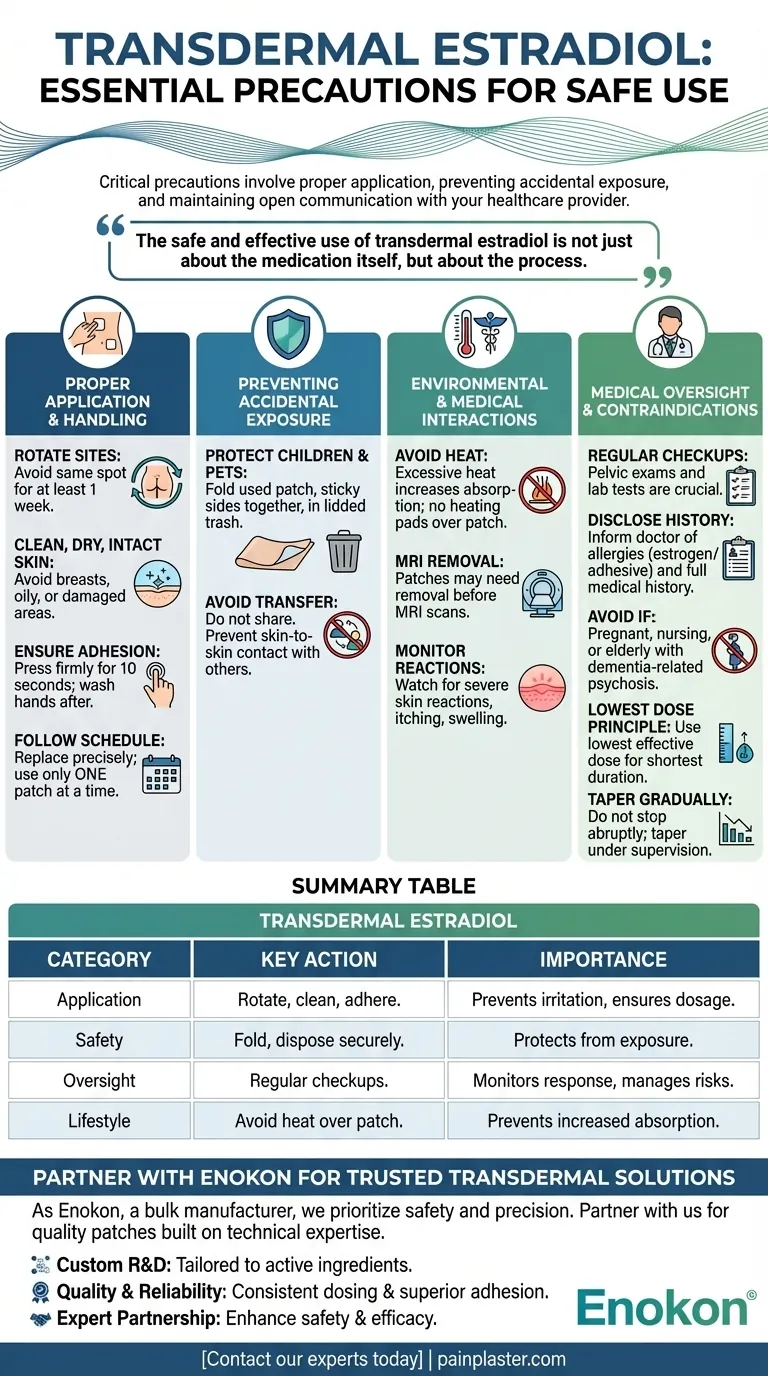
When using transdermal estradiol, the most critical precautions involve proper application, preventing accidental exposure to others, and maintaining open communication with your healthcare provider. You must rotate application sites to avoid skin irritation, apply the patch to clean and unbroken skin, and ensure used patches are disposed of safely where children and pets cannot access them.
The safe and effective use of transdermal estradiol is not just about the medication itself, but about the process. Success depends on meticulous application technique, vigilant awareness of potential risks, and a collaborative partnership with your doctor to monitor your body's response.
Proper Application and Handling
Correct application is the foundation of safe transdermal therapy. Errors at this stage can lead to incorrect dosing, skin reactions, and reduced effectiveness.
Choosing the Right Application Site
Always apply the patch or gel to a clean, dry, and intact area of skin. The lower abdomen or buttocks are common sites.
Critically, avoid applying estradiol to the breasts or to skin that is oily, damaged, irritated, or has been recently shaved.
The Importance of Site Rotation
To prevent skin irritation or allergic reactions, you must rotate the location where you apply the patch. Do not use the same spot for at least one week after a patch has been removed.
Ensuring Correct Adhesion
After applying a new patch, press down firmly with the palm of your hand for about 10 seconds to ensure it is sealed to the skin, especially around the edges. Always wash your hands after handling the patch.
Managing Your Schedule
Follow the prescribed schedule precisely, for instance, replacing the patch every 72 hours. Unless specifically instructed by your doctor, use only one patch at a time.
Preventing Accidental Exposure
A used patch still contains active hormones that can be harmful to others. Preventing accidental exposure is a primary safety responsibility.
Protecting Children and Pets
A discarded patch can be extremely dangerous if ingested by a child or pet. Fold the used patch in half with the sticky sides together and dispose of it in a trash can with a lid.
Avoiding Transfer to Others
The medication should not be shared. Ensure that the application site is not in a place where others, particularly children, might come into prolonged skin-to-skin contact with the patch.
Understanding Environmental and Medical Interactions
External factors and your own medical history can significantly impact how transdermal estradiol works and the risks associated with it.
Factors That Affect Absorption
Be aware that excessive heat can increase hormone absorption, so avoid using heating pads over the patch. Discuss the use of sunscreen on or near the application site with your doctor, as it may affect absorption.
Patches may also need to be removed before an MRI scan, as some contain metal components.
Allergic Reactions and Side Effects
Watch for signs of a severe skin reaction, such as intense itching, swelling, or blistering at the application site. If you experience any other unusual or severe side effects, contact your doctor immediately.
The Role of Regular Monitoring
Consistent medical oversight is crucial. Regular checkups, including pelvic exams and lab tests, allow your doctor to monitor your response to the therapy and adjust as needed.
Key Precautions and Contraindications
This therapy is not suitable for everyone, and its use requires a clear understanding of its limitations and potential risks.
Disclosing Your Full Medical History
Before starting, inform your doctor of any allergies, especially to estrogens or adhesives. It is also critical to disclose your entire medical history.
Who Should Avoid This Medication
Transdermal estradiol should not be used if you are pregnant or nursing. It is also associated with an increased risk of death in elderly patients with dementia-related psychosis.
The Lowest Effective Dose Principle
To minimize long-term risks, treatment should always utilize the lowest effective dose for the shortest duration necessary to achieve your therapeutic goals.
Tapering Off Usage
Do not stop using the medication abruptly. Usage should be tapered gradually under the direct supervision of your healthcare provider.
Making the Right Choice for Your Goal
Applying these precautions correctly ensures you are using this therapy as safely and effectively as possible.
- If your primary focus is starting treatment correctly: Meticulously follow the application and disposal instructions provided with your medication and by your pharmacist.
- If your primary focus is long-term safety: Prioritize regular medical checkups and open communication with your doctor about any changes or side effects you experience.
- If your primary focus is avoiding complications: Be vigilant about rotating application sites, preventing heat exposure to the patch, and disclosing all other medications or supplements you take.
Ultimately, empowering yourself with this knowledge is the best precaution you can take for your health.
Summary Table:
| Precaution Category | Key Action | Why It's Important |
|---|---|---|
| Application | Rotate sites, apply to clean/dry skin, ensure full adhesion. | Prevents skin irritation and ensures correct dosage delivery. |
| Safety | Fold used patches sticky-side together; dispose securely. | Protects children and pets from accidental exposure to active hormones. |
| Medical Oversight | Regular check-ups and open communication with your doctor. | Monitors body's response and manages potential risks or side effects. |
| Lifestyle | Avoid heat over patch (e.g., heating pads). | Prevents increased, unpredictable hormone absorption. |
Need a reliable, high-quality transdermal patch for your hormone therapy product line?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we understand that safety and precision are paramount. We partner with healthcare and pharmaceutical distributors and brands to deliver patches built on technical expertise.
Benefit from our capabilities:
- Custom R&D: We can develop patches tailored to your specific active ingredients and release profiles.
- Quality & Reliability: Our manufacturing processes prioritize consistent dosing and superior adhesion.
- Expert Partnership: Leverage our technical knowledge to enhance your product's safety and efficacy.
Contact our experts today to discuss how we can support the development of your trusted transdermal therapies.
Visual Guide
Related Products
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Menthol Gel Pain Relief Patch
People Also Ask
- How do you apply the Signal Relief patch to find the proper placement? A Step-by-Step Guide to Maximum Relief
- What precautions should be taken with buprenorphine patches? Ensure Safe Use and Avoid Overdose Risks
- Can the pain relief patch be used with other external analgesic products? A Critical Safety Guide
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing
- Can pregnant women use pain relief patches? Your Essential Guide to Safe Pain Management















