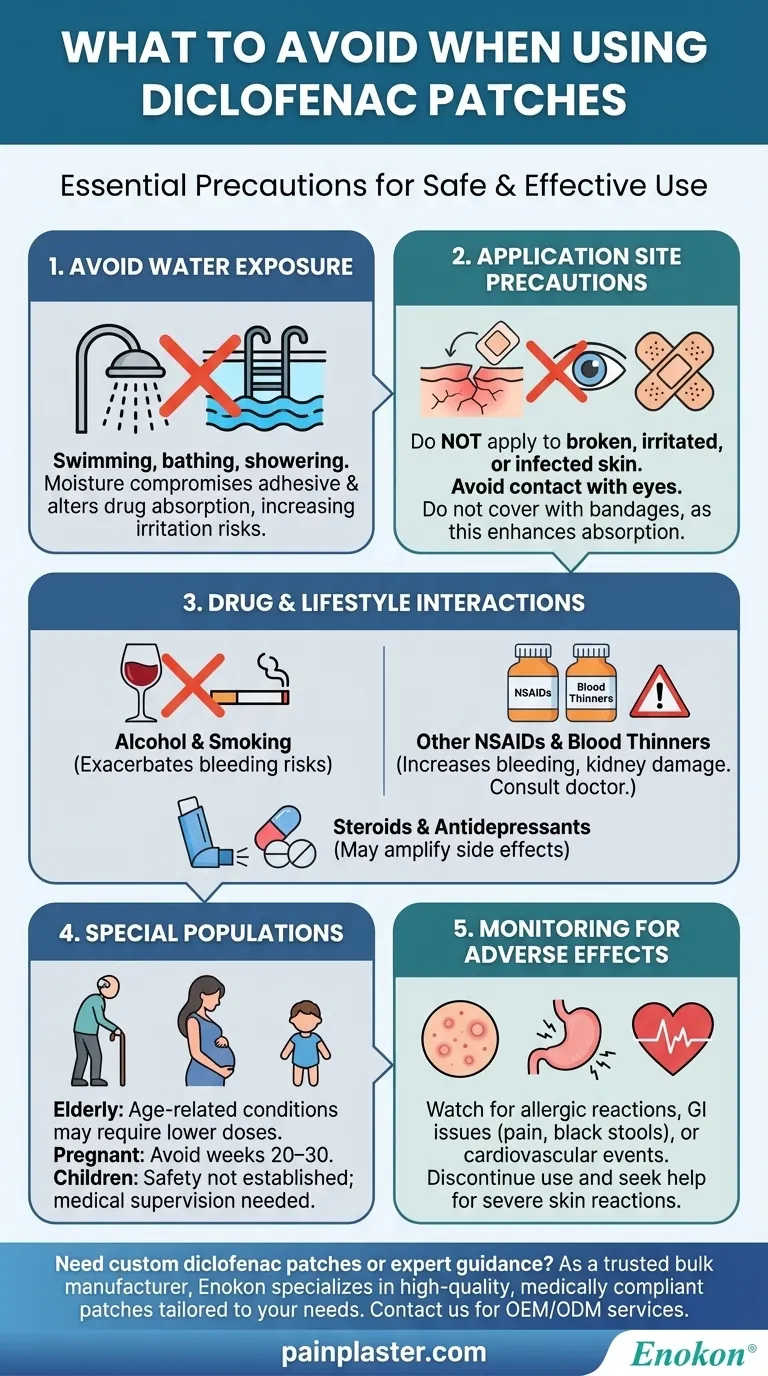
When using pain relief patches like diclofenac, certain precautions must be taken to ensure safety and effectiveness. Key avoidances include exposure to water (swimming, bathing, showering), applying to broken or irritated skin, and combining with other NSAIDs or blood thinners without medical advice. Special populations (e.g., elderly, pregnant women, children) require additional caution due to higher risks of side effects or unestablished safety profiles. Proper application and monitoring for adverse reactions are critical to minimize health risks.

Key Points Explained:
-
Avoid Water Exposure
- Diclofenac patches should never be worn during water activities like swimming, bathing, or showering. Moisture can compromise the adhesive, leading to patch detachment or altered drug absorption.
- Water exposure may also increase skin irritation or systemic absorption, raising the risk of side effects such as stomach bleeding or kidney issues.
-
Application Site Precautions
- Do not apply patches to broken, irritated, or infected skin, as this can lead to excessive drug absorption or localized reactions.
- Avoid contact with eyes; rinse immediately if accidental exposure occurs.
- Covering treated areas with bandages or dressings is discouraged, as it may enhance absorption and cause unintended systemic effects.
-
Drug and Lifestyle Interactions
- Alcohol/Smoking: These can exacerbate bleeding risks, especially when combined with diclofenac’s blood-thinning properties.
- Other NSAIDs: Concurrent use with oral NSAIDs (e.g., ibuprofen) or blood thinners (e.g., warfarin) increases the likelihood of gastrointestinal bleeding or kidney damage. Always consult a doctor before combining medications.
- Steroids/Antidepressants: These may interact with diclofenac, amplifying side effects like dizziness or stomach ulcers.
-
Special Populations
- Elderly: Age-related kidney or heart conditions may necessitate lower doses or alternative treatments.
- Pregnant Women: Avoid use between weeks 20–30 of pregnancy due to potential harm to fetal development.
- Children: Safety and efficacy are not established for certain formulations; pediatric use should be medically supervised.
-
Monitoring for Adverse Effects
- Watch for signs of allergic reactions (e.g., rash, swelling), gastrointestinal issues (e.g., stomach pain, black stools), or cardiovascular events (e.g., chest pain).
- Discontinue use and seek medical help if severe skin reactions (e.g., peeling, blisters) or dizziness occur.
By adhering to these guidelines, users can mitigate risks while benefiting from diclofenac patches’ therapeutic effects. Always follow healthcare provider instructions for optimal outcomes.
Summary Table:
| Precaution | Reason |
|---|---|
| Avoid water exposure | Compromises adhesion, alters drug absorption, increases irritation risks. |
| Skip broken/irritated skin | Prevents excessive absorption or localized reactions. |
| No concurrent NSAIDs/blood thinners | Reduces risk of bleeding, kidney damage, or stomach ulcers. |
| Monitor elderly/pregnant users | Higher susceptibility to side effects; requires medical supervision. |
| Watch for adverse reactions | Early detection of allergies, skin issues, or cardiovascular events. |
Need custom diclofenac patches or expert guidance?
As a trusted bulk manufacturer of transdermal pain relief solutions, Enokon specializes in high-quality, medically compliant patches tailored to your needs. Whether you're a healthcare distributor or a brand seeking reliable OEM/ODM services, our technical expertise ensures safe, effective formulations. Contact us today to discuss R&D support, regulatory compliance, or bulk orders!
Visual Guide
Related Products
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Natural Herbal Wormwood Patch Pain Plaster
People Also Ask
- What side effects might occur from using capsaicin patches? Understand the difference between normal sensation and danger signs.
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing
- Can pregnant women use pain relief patches? Your Essential Guide to Safe Pain Management
- How do you apply the Signal Relief patch to find the proper placement? A Step-by-Step Guide to Maximum Relief
















