When considering topical lidocaine during pregnancy or breastfeeding, the simple answer is that its effects are not definitively known. Because of this, use is generally not recommended unless a healthcare provider determines it is clearly needed. Consulting your doctor before use is not just a suggestion—it is the essential first step to ensure safety.
The core issue is a lack of specific data. Because the effects of topical lidocaine on a developing fetus or a nursing infant have not been formally studied, any use becomes a calculation of benefit versus unknown risk—a decision that must be guided by a medical professional.

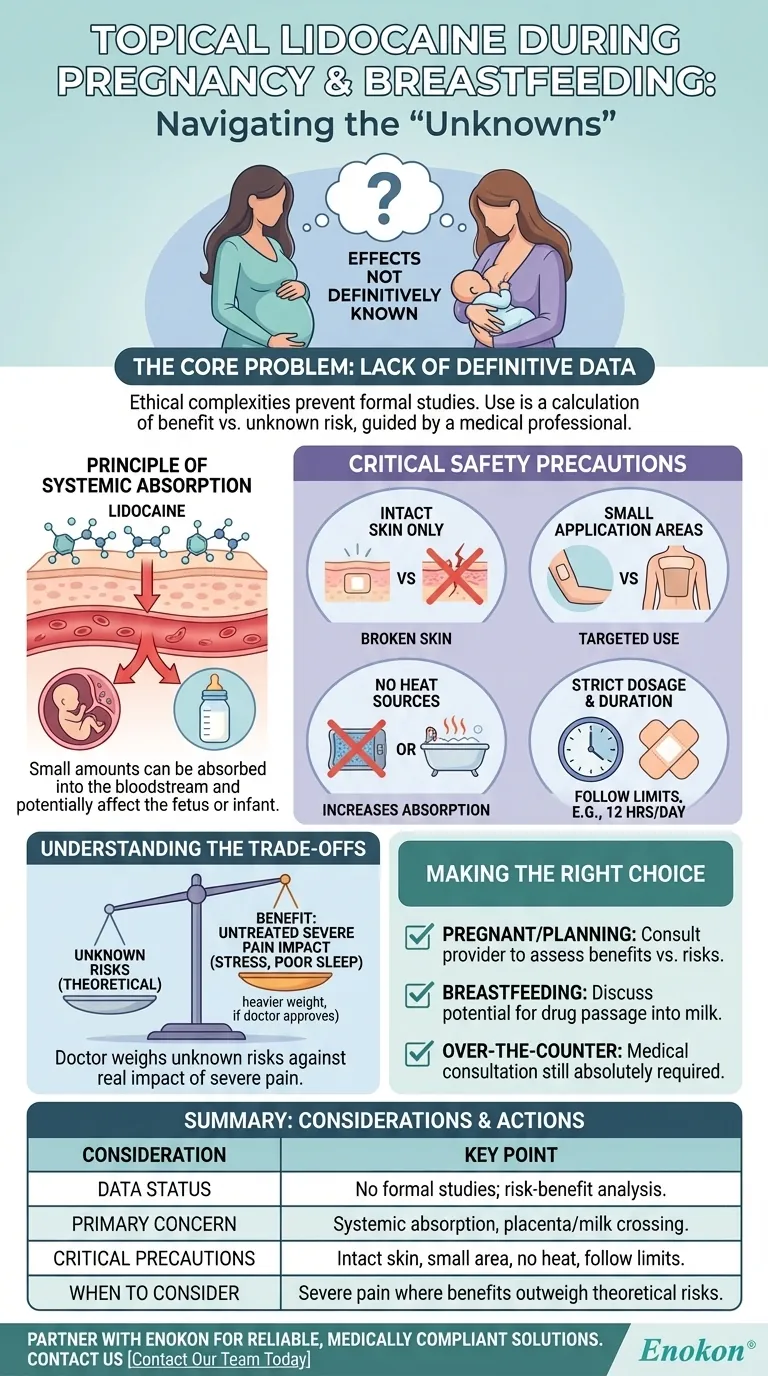
The Core Problem: A Lack of Definitive Data
The cautious stance on topical lidocaine during pregnancy and breastfeeding stems from a fundamental principle in medicine: without clear evidence of safety, one must assume a potential for risk.
Why the Effects are "Unknown"
Conducting clinical trials on pregnant or breastfeeding individuals is ethically complex and therefore rare. This results in a lack of direct scientific data for many medications, including topical lidocaine, leaving medical guidance to rely on general principles of pharmacology.
The Principle of Systemic Absorption
Even though lidocaine is applied topically to the skin, a small amount can be absorbed into the bloodstream. This process is called systemic absorption. Once in the bloodstream, the drug could potentially cross the placenta to a fetus or pass into breast milk.
The Necessity of Medical Supervision
A healthcare provider can assess your unique situation. They will consider the specific location and severity of your pain, the stage of your pregnancy or nursing, and the lowest effective dose to make an informed risk-benefit analysis that you cannot make on your own.
General Safety Precautions That Become Critical
While the following are standard precautions for any user, they take on heightened importance during pregnancy and breastfeeding to minimize the primary concern: systemic absorption.
Use Only on Intact, Unbroken Skin
Applying lidocaine to broken, cut, or irritated skin dramatically increases the amount of medication that enters your bloodstream. This significantly elevates potential risk.
Avoid Large Application Areas
The larger the surface area of skin you cover, the greater the total amount of lidocaine that can be absorbed into your body. Use the smallest possible amount on the most targeted area.
Steer Clear of Heat Sources
Never apply heat, such as from a heating pad or hot bath, to an area treated with topical lidocaine. Heat increases blood flow to the skin, which can accelerate the rate and amount of drug absorption.
Strictly Adhere to Dosage and Duration
Follow instructions precisely. For instance, over-the-counter patches (like a 4% lidocaine patch) should typically not be worn for more than 12 hours a day. Overuse increases the total absorbed dose over time.
Understanding the Trade-offs
The decision to use topical lidocaine is not always a simple "no." Your doctor's role is to weigh the unknown, but likely low, risk against the very real impact of untreated pain.
When Use Might Be "Clearly Needed"
Severe pain during pregnancy can have its own negative health consequences, such as increased stress, high blood pressure, or poor sleep. In some cases, a doctor may decide that the benefit of targeted, temporary pain relief outweighs the theoretical risks of controlled, proper use of topical lidocaine.
The Irreplaceable Role of Your Provider
Your healthcare provider is the only person qualified to make this judgment call. They can consider all factors, discuss potential alternatives, and provide a personalized recommendation that prioritizes the health of both you and your child.
Making the Right Choice for Your Situation
Navigating medication use during this time requires a partnership with your healthcare team. The final decision should always be a shared one, based on clear medical guidance.
- If you are pregnant or planning to become pregnant: You must consult your healthcare provider before using topical lidocaine to assess if the benefits outweigh the unknown risks for your specific condition.
- If you are currently breastfeeding: Speak with your doctor, who can help you understand the potential for the drug to pass into breast milk and decide on the safest course of action.
- If you are considering an over-the-counter product: Do not assume "over-the-counter" means risk-free during pregnancy or breastfeeding; the absolute requirement for medical consultation still applies.
Ultimately, making an informed choice in collaboration with your doctor is the best way to manage your health while protecting your child.
Summary Table:
| Consideration | Key Point |
|---|---|
| Data Status | No formal studies on fetal/infant effects; use is based on risk-benefit analysis. |
| Primary Concern | Systemic absorption can allow lidocaine to cross placenta or enter breast milk. |
| Critical Precautions | Apply only to intact skin, avoid large areas/heat, and follow dosage limits strictly. |
| When Use Might Be Considered | If pain is severe and a doctor deems benefits outweigh theoretical risks. |
Need reliable, medically compliant topical pain relief solutions?
As Enokon, a bulk manufacturer of trusted transdermal patches and pain plasters, we partner with healthcare and pharmaceutical distributors to ensure product safety and efficacy. Our technical expertise supports custom R&D for formulations that meet strict medical guidelines.
Contact our team today to discuss how we can help you develop safe, targeted pain management products for sensitive patient populations.
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Natural Herbal Wormwood Patch Pain Plaster
People Also Ask
- How is the lidocaine patch administered? A Step-by-Step Guide for Safe & Effective Pain Relief
- What systemic side effects can lidocaine patches cause? Minimizing Risks for Safe Pain Relief
- How can you use lidocaine patches for multiple sore spots? A Guide to Safe, Effective Pain Relief
- How are lidocaine patches typically used for pain relief during pregnancy? A Guide to Safe, Targeted Relief
- When should someone contact a doctor regarding lidocaine patch use? Ensure Safe Pain Relief
















