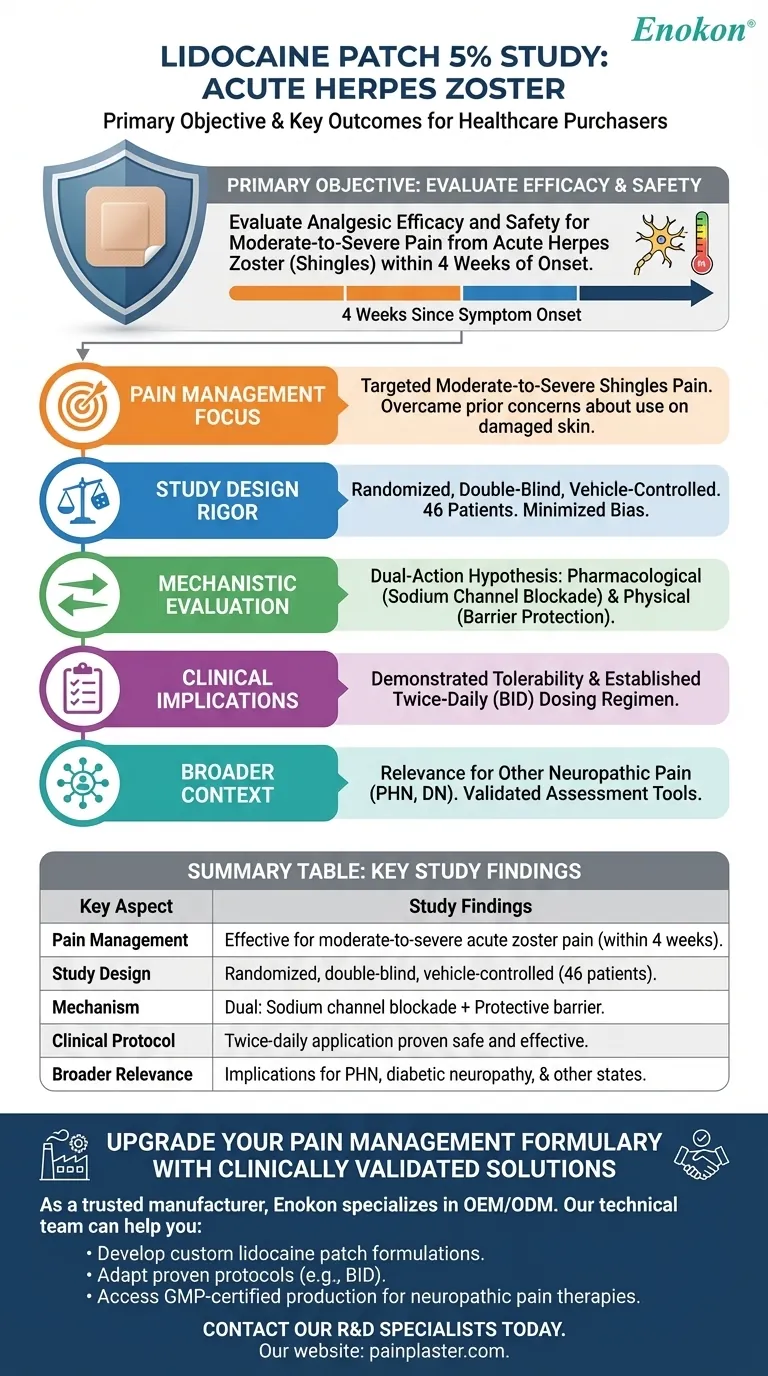
The primary objective of the study on lidocaine patch 5 percent was to evaluate its analgesic efficacy and safety in treating moderate to severe pain associated with acute herpes zoster (shingles). This investigation was significant because prior concerns about skin damage had limited its use in this condition. The study employed a rigorous randomized, double-blind, vehicle-controlled design involving 46 patients with recent-onset herpes zoster, ultimately demonstrating the patch's effectiveness and tolerability when applied twice daily.
Key Points Explained:
-
Pain Management Focus
- Targeted acute herpes zoster pain (moderate-to-severe intensity) within 4 weeks of symptom onset
- Addressed a critical gap: prior avoidance due to theoretical risks to compromised skin
-
Study Design Rigor
- Randomized, double-blind methodology minimized bias
- Vehicle-controlled parallel groups ensured reliable comparisons
- 46-patient cohort provided statistically meaningful data
-
Mechanistic Evaluation
- Assessed dual-action hypothesis:
- Pharmacological: Lidocaine’s sodium channel blockade
- Physical: Barrier protection for sensitized skin
- Twice-daily application protocol balanced efficacy with practicality
- Assessed dual-action hypothesis:
-
Clinical Implications
- Demonstrated tolerability in damaged skin populations
- Established dosing regimen (BID application) for acute zoster pain
- Expanded evidence for topical analgesics in neuropathic conditions
-
Broader Context
- Findings potentially relevant to other neuropathic pain states (e.g., PHN, DN)
- Validated assessment tools like NPS for future research
The study’s outcomes quietly revolutionized localized pain management, proving that even damaged skin could benefit from targeted topical therapies. For healthcare purchasers, these results justify stocking lidocaine patches as a frontline option for shingles-related pain control.
Summary Table:
| Key Aspect | Study Findings |
|---|---|
| Pain Management | Effective for moderate-to-severe acute herpes zoster pain within 4 weeks onset |
| Study Design | Randomized, double-blind, vehicle-controlled trial with 46 patients |
| Mechanism | Dual action: sodium channel blockade + protective barrier for sensitized skin |
| Clinical Protocol | Twice-daily application proven safe and effective |
| Broader Relevance | Implications for PHN, diabetic neuropathy, and other neuropathic pain states |
Upgrade your pain management formulary with clinically validated solutions
As a trusted manufacturer of transdermal pain relief systems, Enokon specializes in OEM/ODM partnerships for healthcare distributors and brands. Our technical team can help you:
- Develop custom lidocaine patch formulations with optimized drug delivery
- Adapt proven protocols (like BID application) for your product line
- Access GMP-certified production for neuropathic pain therapies
Contact our R&D specialists today to discuss compliant, study-backed transdermal solutions.
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How does the lidocaine patch work? Targeted Relief for Nerve Pain Explained
- How are lidocaine patches typically used for pain relief during pregnancy? A Guide to Safe, Targeted Relief
- When should someone contact a doctor regarding lidocaine patch use? Ensure Safe Pain Relief
- For what condition are lidocaine patches approved in the United Kingdom? A Guide to Postherpetic Neuralgia Treatment
- Is it safe to use lidocaine patches while breastfeeding? Expert Guidance for Nursing Mothers

















