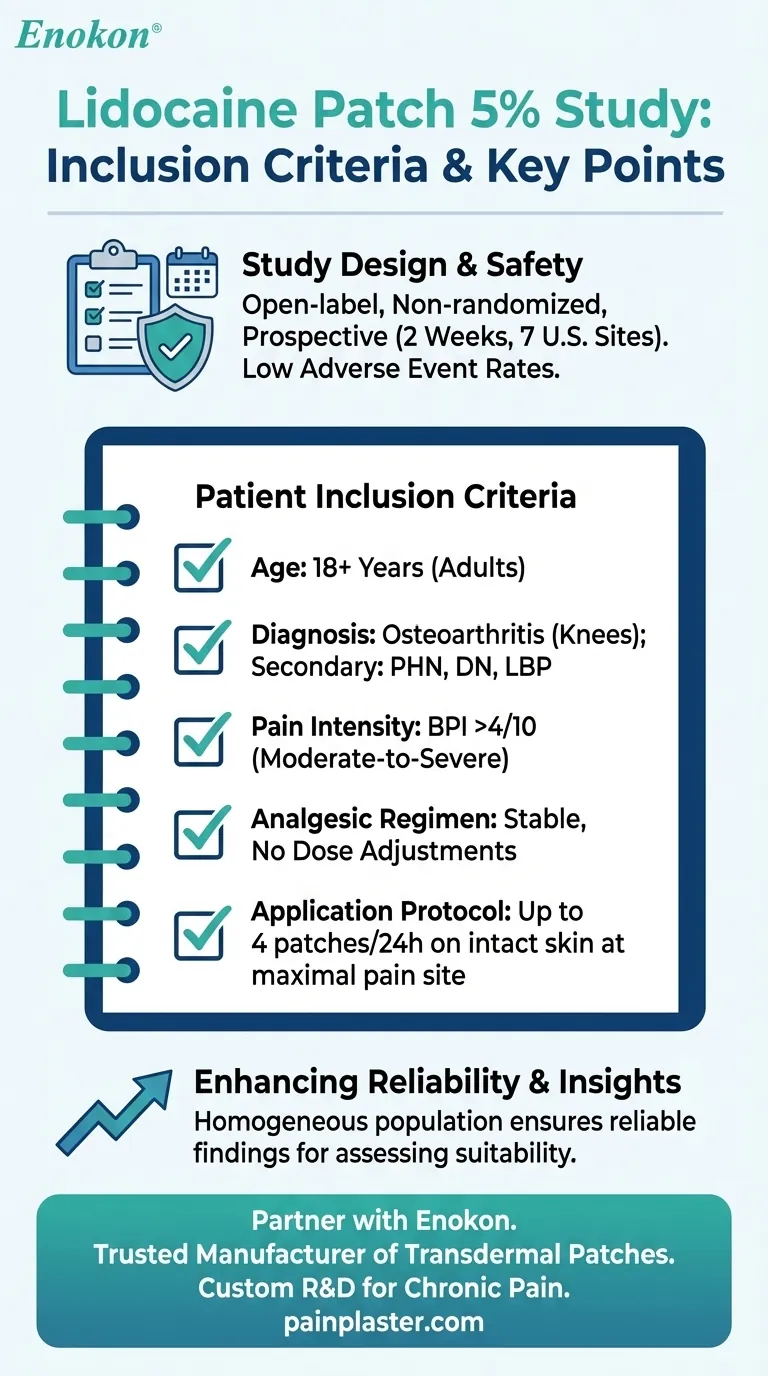
The inclusion criteria for patients in the lidocaine patch 5 percent study were designed to select individuals with specific pain conditions and stable analgesic regimens. Participants had to be adults aged 18 or older with osteoarthritis in one or both knees, maintaining a consistent pain management plan, and reporting average daily pain intensity above 4/10 on the Brief Pain Inventory (BPI). The study focused on evaluating the patch's impact on pain qualities in conditions like postherpetic neuralgia (PHN), diabetic neuropathy (DN), and low-back pain (LBP), using the Neuropathic Pain Scale (NPS). The trial was open-label, non-randomized, and prospective, conducted over two weeks across multiple U.S. sites, with patients applying up to four patches daily to areas of maximal pain.
Key Points Explained:
-
Age and Gender Criteria:
- Patients had to be 18 years or older, encompassing both men and women. This ensures the study results are applicable to the adult population most likely to use the patch clinically.
-
Diagnosis Requirements:
- Participants needed a confirmed diagnosis of osteoarthritis in one or both knees. This specificity helps target the study’s focus on joint-related chronic pain.
- Secondary conditions like PHN, DN, or LBP were also evaluated, broadening the scope to neuropathic and musculoskeletal pain.
-
Pain Intensity Threshold:
- A BPI score >4/10 for average daily pain was mandatory. This cutoff identifies patients with moderate-to-severe pain, ensuring the patch’s efficacy is tested in clinically relevant scenarios.
-
Stable Analgesic Regimen:
- Patients were required to maintain their existing pain management plans without dose adjustments. This controls for confounding variables, isolating the patch’s additive effect.
-
Application Protocol:
- Patches were applied to intact skin (no blisters) at the site of maximal pain, with a limit of four patches per 24 hours. This standardizes treatment while mimicking real-world use.
-
Study Design:
- The open-label, non-randomized, prospective design across seven U.S. sites provides pragmatic insights, though it may lack the rigor of blinded trials. The 2-week duration balances practicality with sufficient time to observe effects.
-
Safety and Tolerability:
- Both active and vehicle patches showed low adverse event rates, supporting the patch’s safety profile—a key consideration for purchasers evaluating risk-benefit ratios.
By adhering to these criteria, the study ensured a homogeneous patient population, enhancing the reliability of findings for healthcare providers and purchasers assessing the patch’s suitability for chronic pain management. Would the inclusion of a broader pain scale, like the NPS, help tailor the patch’s use to specific pain subtypes in future research?
Summary Table:
| Criteria | Requirement |
|---|---|
| Age | 18 years or older |
| Diagnosis | Osteoarthritis in one/both knees; secondary conditions (PHN, DN, LBP) evaluated |
| Pain Intensity (BPI) | >4/10 average daily pain |
| Analgesic Regimen | Stable, no dose adjustments |
| Patch Application | Up to 4 patches/24 hours on intact skin at maximal pain site |
| Study Design | Open-label, non-randomized, prospective (2 weeks, 7 U.S. sites) |
| Safety Profile | Low adverse event rates for active/vehicle patches |
Need clinically validated pain relief solutions? Partner with Enokon, a trusted bulk manufacturer of transdermal patches and pain plasters for healthcare distributors and pharma brands. Our expertise in custom R&D ensures tailored formulations for chronic pain management. Contact us to discuss how our lidocaine patches can meet your patients' needs with proven safety and efficacy.
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How should the treated area be protected while wearing a lidocaine patch? Safety Tips for Effective Pain Relief
- What systemic side effects can lidocaine patches cause? Minimizing Risks for Safe Pain Relief
- How does the lidocaine patch work? Targeted Relief for Nerve Pain Explained
- How are lidocaine patches typically used for pain relief during pregnancy? A Guide to Safe, Targeted Relief
- For what condition are lidocaine patches approved in the United Kingdom? A Guide to Postherpetic Neuralgia Treatment













