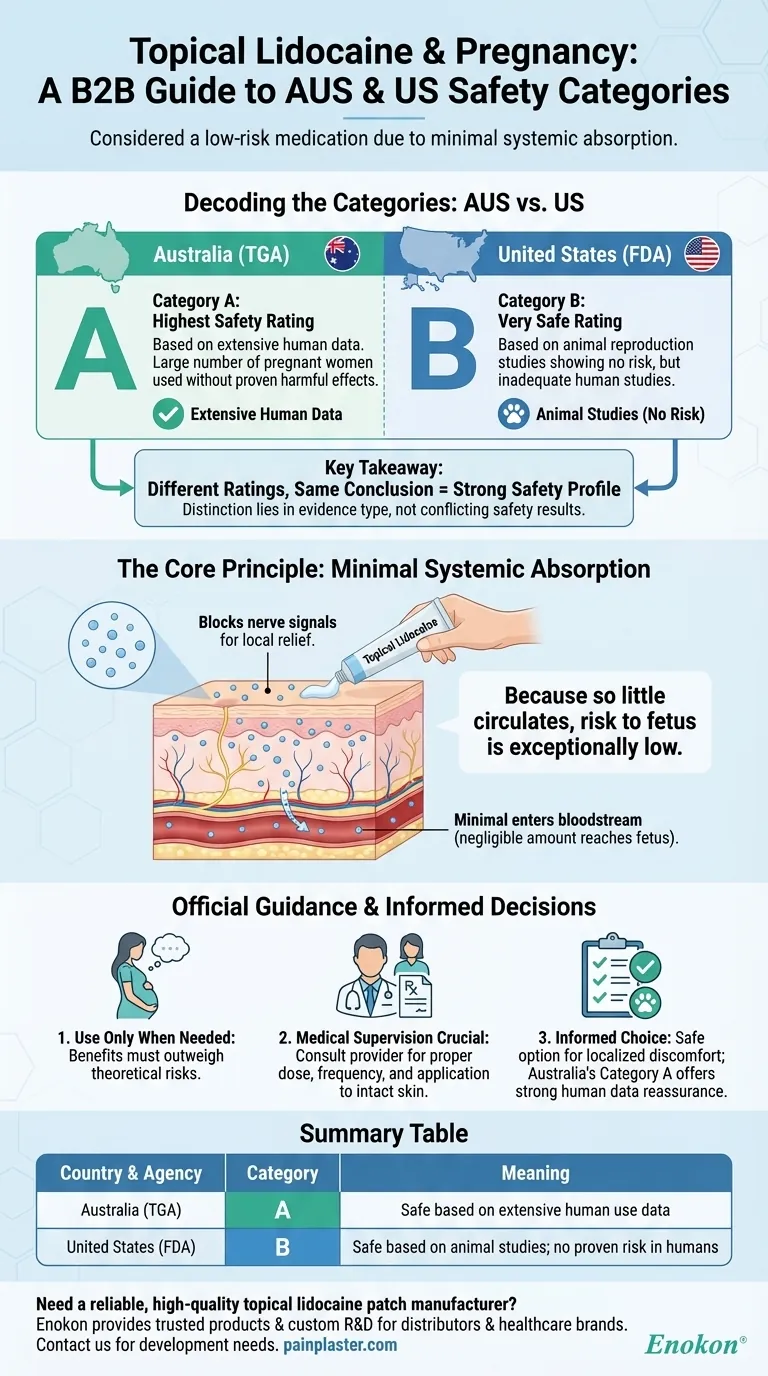
In Australia and the US, topical lidocaine is considered a low-risk medication during pregnancy. In Australia, it is classified as Therapeutic Goods Administration (TGA) Pregnancy Category A. In the United States, it is classified as Food and Drug Administration (FDA) Pregnancy Category B. Both categories indicate a strong safety profile, though they are based on different types of evidence.
The key takeaway is that the difference between Australia's Category A and the US's Category B is not about conflicting safety results, but rather the nature of the data used for classification. The consensus is that when applied topically as directed, very little lidocaine enters the bloodstream, making the risk to a developing fetus exceptionally low.

Decoding the Pregnancy Categories: A vs. B
The official ratings from different national health agencies can seem confusing, but they point to the same conclusion. The distinction lies in the type of evidence each system prioritizes.
Australia's TGA Category A Explained
Category A is the highest safety rating provided by Australia's TGA.
It means the medication has been taken by a large number of pregnant women and women of childbearing age without any proven increase in the frequency of malformations or other direct or indirect harmful effects on the fetus. This rating is based on extensive human data from real-world use over many years.
The US FDA's Category B Explained
Category B is also considered a very safe rating by the US FDA.
This classification means that animal reproduction studies have failed to demonstrate a risk to the fetus, but there are no adequate and well-controlled studies in pregnant women. Many common and safely used medications, like paracetamol (acetaminophen), fall into this category.
Why is There a Difference?
The different ratings do not reflect a disagreement on safety. They are a product of two distinct regulatory frameworks.
Australia's Category A is heavily influenced by a long history of safe use in the human population (post-market surveillance). The US system, historically, placed a greater emphasis on the results of formal, controlled animal and human trials, which are often not conducted in pregnant women for ethical reasons.
The Core Principle: Systemic Absorption
To understand why topical lidocaine is considered safe, the most important concept is systemic absorption—how much of the drug actually enters your bloodstream.
How Topical Lidocaine Works
Lidocaine is a local anesthetic. When applied to the skin, its job is to block nerve signals in that immediate area, providing temporary numbness and pain relief.
The Key Factor is Minimal Absorption
When applied correctly to a small area of intact skin, only a very small fraction of the lidocaine passes through the skin into the bloodstream.
Because so little of the medication circulates throughout the body, the amount that could potentially reach the fetus is considered negligible and highly unlikely to cause harm.
Understanding the Official Guidance
Even with safe medications, you will always find cautionary language regarding use during pregnancy. This is a standard and responsible medical practice.
The "Use Only When Clearly Needed" Caveat
This advice is standard for nearly all medications during pregnancy. It encourages a thoughtful approach where the benefit of using the medication (e.g., pain relief that improves quality of life) must clearly outweigh any theoretical risk, however small.
The Importance of Medical Supervision
The recommendation to consult a healthcare provider is crucial. A doctor or pharmacist can assess your specific situation, confirm that topical lidocaine is the right choice, and ensure you understand the proper application to minimize absorption.
This includes guidance on how much to use, how often, and avoiding application on broken or irritated skin, which can increase the amount of drug that enters your system.
Making an Informed Decision
Navigating medication use during pregnancy requires balancing comfort and safety. Here’s how to approach the use of topical lidocaine based on its strong safety profile.
- If your primary focus is reassurance from human data: Australia's Category A rating provides strong evidence from its use by a large number of pregnant women without observed harm.
- If you require occasional use for localized discomfort: Topical lidocaine is widely regarded as a safe option due to its minimal systemic absorption, but always confirm with your doctor first.
- If you are weighing it against other options: Given its safety ratings in both countries, topical lidocaine is considered one of the safer local anesthetic choices available for use during pregnancy.
Ultimately, a conversation with your healthcare provider is the definitive step to ensure both your well-being and your baby's safety.
Summary Table:
| Country | Agency | Pregnancy Category | Meaning |
|---|---|---|---|
| Australia | TGA | A | Safe based on extensive human use data |
| United States | FDA | B | Safe based on animal studies; no proven risk in humans |
Need a reliable, high-quality topical lidocaine patch? As Enokon, a bulk manufacturer of transdermal patches and pain plasters, we provide pharmaceutical distributors and healthcare brands with trusted, consistent products. Our technical expertise supports custom R&D to meet your specific requirements. Contact our team today to discuss your development needs.
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
People Also Ask
- How is the lidocaine patch administered? A Step-by-Step Guide for Safe & Effective Pain Relief
- How should the treated area be protected while wearing a lidocaine patch? Safety Tips for Effective Pain Relief
- How are lidocaine patches typically used for pain relief during pregnancy? A Guide to Safe, Targeted Relief
- How does the lidocaine patch work? Targeted Relief for Nerve Pain Explained
- Are lidocaine patches safe to use during pregnancy? A Guide to Making an Informed Choice
















