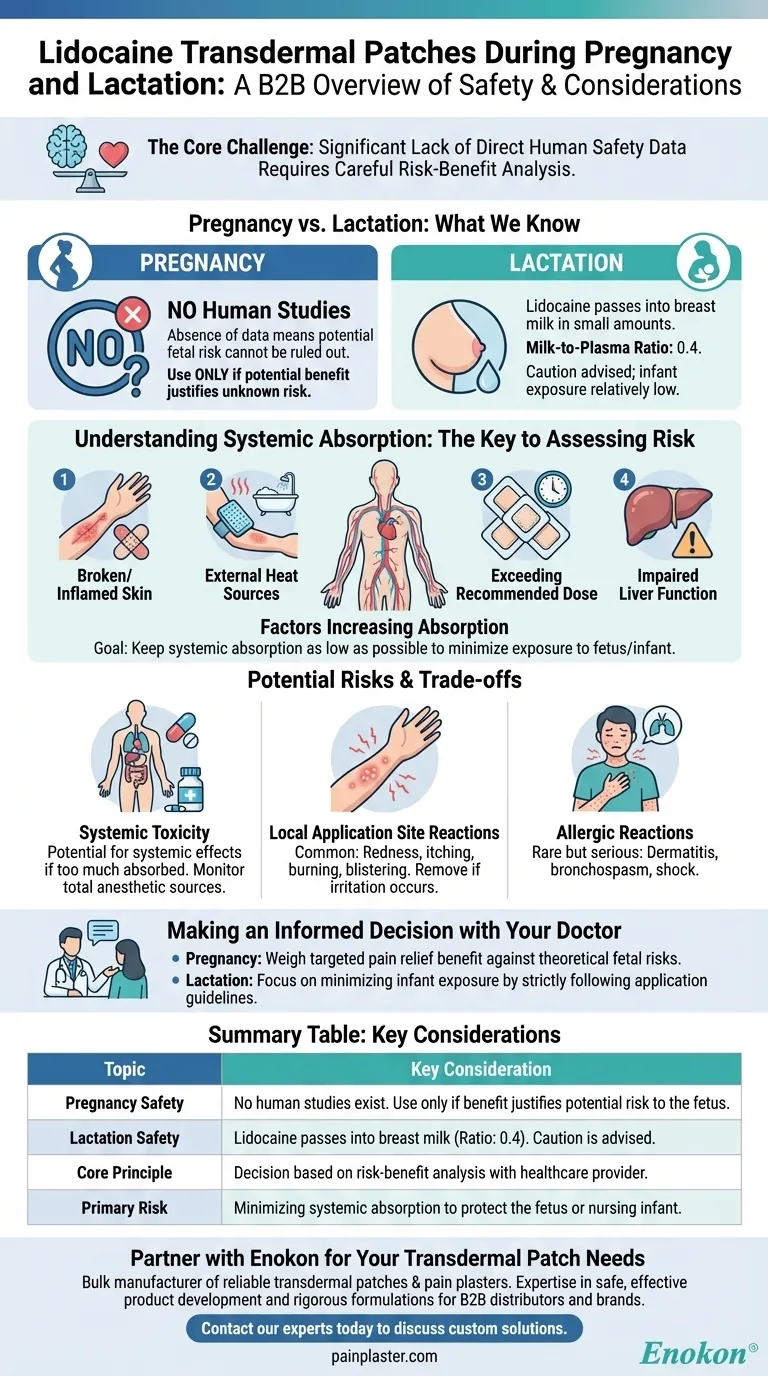
When considering the use of lidocaine transdermal patches during pregnancy or lactation, the defining factor is a significant lack of direct human safety data. For pregnancy, no studies in women exist. For lactation, we know that lidocaine is excreted into breast milk in small amounts, but caution is still advised. Therefore, any use must be a carefully considered decision between you and your healthcare provider.
The central challenge is not that lidocaine patches are proven dangerous, but that their safety profile in pregnancy is unknown. The decision to use them hinges on a critical risk-benefit analysis, focusing on minimizing systemic absorption to protect the fetus or nursing infant.

The Core Issue: Lack of Human Data
The primary guidance from health authorities is rooted in a deliberate and cautious approach when definitive human studies are unavailable. This is common for many medications concerning pregnancy and lactation.
The Absence of Pregnancy Studies
There are no adequate and well-controlled studies of lidocaine transdermal patch use in pregnant women. This absence of data means a potential risk to the fetus cannot be ruled out.
Because of this, the standard medical advice is to use the patch during pregnancy only if the potential benefit justifies the potential, albeit unknown, risk to the fetus.
Data Available for Lactation
For breastfeeding mothers, the situation is slightly clearer. Lidocaine is known to pass into human milk.
However, the data shows a milk-to-plasma ratio of 0.4. This means the concentration of lidocaine in breast milk is less than half the concentration in the mother's blood, suggesting a relatively low level of exposure for the infant.
Understanding Systemic Absorption
The key to assessing the risk is understanding how much lidocaine enters your bloodstream. The patch is designed for local action, but some of the drug will always be absorbed systemically. The goal is to keep this absorption as low as possible.
Factors that Increase Absorption
Several factors can significantly increase the amount of lidocaine that enters your system, thereby increasing potential exposure to a fetus or nursing infant.
- Applying to broken or inflamed skin: Damaged skin absorbs medication much more readily than intact skin.
- Using external heat sources: Applying heating pads, electric blankets, or even taking a hot bath can increase blood flow to the area and enhance drug absorption.
- Exceeding the recommended dose: Using more patches than prescribed, or leaving them on for longer than directed, directly increases the total dose absorbed.
- Impaired liver function: Since the liver helps eliminate lidocaine from the body, severe hepatic impairment can lead to higher-than-expected concentrations in the blood.
Potential Risks and Trade-offs
Beyond the specific concerns for pregnancy and lactation, it is important to be aware of the general risks associated with lidocaine patches for any user.
Systemic Toxicity
If too much lidocaine is absorbed, it can cause systemic effects. When used with other products containing local anesthetics, the total amount of absorbed drug from all sources must be carefully monitored to prevent reaching toxic levels.
Local Application Site Reactions
The most common side effects occur where the patch is applied. These can include redness, itching, burning, bruising, blisters, or changes in skin pigmentation. If irritation occurs, the patch should be removed.
Allergic Reactions
Though rare, serious allergic and anaphylactoid reactions can occur. These may involve dermatitis, bronchospasm, shortness of breath, or in severe cases, shock.
Making an Informed Decision with Your Doctor
The choice to use a lidocaine transdermal patch during pregnancy or lactation is a medical decision that requires a thoughtful conversation with your healthcare provider.
- If your primary focus is managing severe, localized pain during pregnancy: The discussion will weigh the benefit of targeted pain relief against the theoretical risks of fetal exposure from minimal systemic absorption.
- If your primary focus is pain management while breastfeeding: The data is more reassuring, and the conversation will likely center on strategies to minimize infant exposure by strictly following application guidelines.
Ultimately, a collaborative decision with your healthcare provider, grounded in these principles of risk management, is the safest path forward.
Summary Table:
| Topic | Key Consideration |
|---|---|
| Pregnancy Safety | No human studies exist. Use only if benefit justifies potential risk to the fetus. |
| Lactation Safety | Lidocaine passes into breast milk in small amounts (milk-to-plasma ratio: 0.4). Caution is advised. |
| Core Principle | Decision must be based on a risk-benefit analysis with your healthcare provider. |
| Primary Risk | Minimizing systemic absorption to protect the fetus or nursing infant is the goal. |
Partner with Enokon for Your Transdermal Patch Needs
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharmaceutical distributors and brands with the technical expertise necessary for safe and effective product development. If you are developing a pain management solution that requires rigorous formulation and a deep understanding of systemic absorption profiles, our custom R&D team is here to help.
Contact our experts today to discuss how we can support your product development with high-quality, custom transdermal solutions.
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heat Relief Capsicum Patch for Lower Back Pain Relief
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
People Also Ask
- How are lidocaine patches typically used for pain relief during pregnancy? A Guide to Safe, Targeted Relief
- For what condition are lidocaine patches approved in the United Kingdom? A Guide to Postherpetic Neuralgia Treatment
- Is it safe to use lidocaine patches while breastfeeding? Expert Guidance for Nursing Mothers
- When should someone contact a doctor regarding lidocaine patch use? Ensure Safe Pain Relief
- How should the treated area be protected while wearing a lidocaine patch? Safety Tips for Effective Pain Relief
















