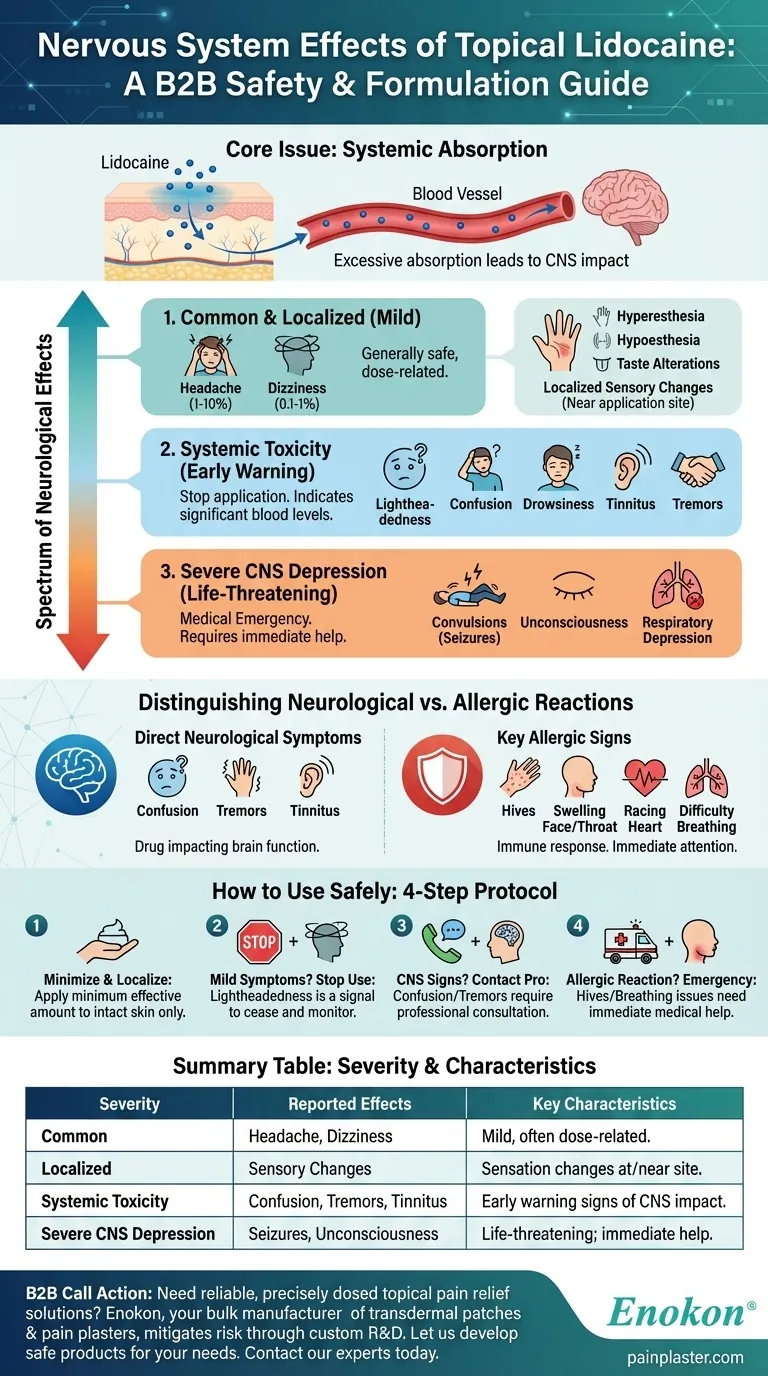
The most common nervous system effects reported with topical lidocaine use are headache and dizziness. However, a broader range of effects, from mild sensory changes to severe central nervous system depression, is possible, typically occurring when the medication is absorbed excessively into the bloodstream.
While topical lidocaine is generally safe for localized use, its nervous system side effects are directly related to systemic absorption. Significant neurological symptoms are a critical warning sign that the dose is too high or it is entering the bloodstream too quickly.

A Spectrum of Neurological Effects
The impact of topical lidocaine on the nervous system exists on a continuum, directly correlated with how much of the drug enters your systemic circulation.
Common and Expected Effects
The most frequently reported effects are generally mild.
- Headache: Occurs in an estimated 1-10% of users.
- Dizziness: A less common effect, reported in 0.1-1% of users.
Localized Sensory Changes
These effects often relate to the medication's direct impact on nerves in and around the application area.
Postmarketing reports have noted hyperesthesia (increased sensitivity), hypoesthesia (reduced sensitivity or numbness), and alterations in taste.
Signs of Systemic Toxicity
When lidocaine is absorbed into the bloodstream in sufficient amounts, it begins to affect the central nervous system (CNS). These are early warning signs.
Symptoms include lightheadedness, confusion, drowsiness, tinnitus (ringing in the ears), and tremors. Their appearance indicates the need to cease application.
Severe Central Nervous System Depression
At dangerously high levels of systemic absorption, lidocaine can cause severe and life-threatening neurological events.
These include convulsions (seizures), unconsciousness, and ultimately, respiratory depression, where the brain's control over breathing is compromised. These are medical emergencies.
Understanding the Core Issue: Systemic Absorption
The fundamental risk with topical lidocaine is the drug moving from its intended local target into the entire body via the bloodstream.
How Topical Becomes Systemic
Lidocaine works by blocking local nerve signals. This effect is desirable at the application site but problematic for the central nervous system.
Excessive absorption can occur if you apply too much product, use it on broken or irritated skin, or cover a large surface area.
The Dose-Dependent Relationship
The risk and severity of all nervous system side effects increase as the concentration of lidocaine in the blood rises.
What begins as mild lightheadedness can progress to seizures if the systemic level continues to climb.
Distinguishing Neurological Effects from Allergic Reactions
It is crucial to differentiate between a direct nervous system side effect and a separate, but equally serious, allergic reaction. Dizziness can be a symptom of both.
Direct Neurological Symptoms
These effects are caused by the drug directly impacting brain function. The key indicators are confusion, tremors, drowsiness, and tinnitus.
Key Signs of an Allergic Reaction
An allergy is an immune system response. The hallmark signs are different and require immediate medical attention.
Look for hives, swelling of the face or throat, a racing heart, and difficulty breathing or swallowing. While dizziness can occur, it will be in the context of these other systemic symptoms.
How to Use Lidocaine Safely
Your approach should be guided by the symptoms you observe, as they provide clear signals about the body's response.
- If you are using lidocaine for minor, localized pain: Apply the minimum effective amount only to intact skin and monitor for any changes in sensation at the site.
- If you experience mild dizziness or lightheadedness: This is a signal of potential systemic absorption. Stop using the product and monitor your symptoms.
- If you observe confusion, tremors, or tinnitus: Cease use immediately and contact a healthcare professional, as these are clear signs of central nervous system toxicity.
- If you develop hives, swelling, or difficulty breathing: This is a potential medical emergency indicating a severe allergic reaction that requires immediate medical help.
Understanding these distinctions allows for the safe use of topical lidocaine while being prepared to recognize critical warning signs.
Summary Table:
| Severity Level | Reported Nervous System Effects | Key Characteristics |
|---|---|---|
| Common | Headache, Dizziness | Mild, often dose-related. |
| Localized | Hyperesthesia, Hypoesthesia, Taste Alterations | Changes in sensation at/near application site. |
| Systemic Toxicity | Lightheadedness, Confusion, Drowsiness, Tinnitus, Tremors | Early warning signs of CNS impact; stop use. |
| Severe CNS Depression | Seizures, Unconsciousness, Respiratory Depression | Life-threatening; requires immediate medical help. |
Need a reliable, precisely dosed topical pain relief solution? As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we help healthcare and pharma distributors and brands mitigate risks through our technical expertise in custom R&D and development. Let us develop a safe and effective product for your needs. Contact our experts today to discuss your requirements.
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Heating Pain Relief Patches for Menstrual Cramps
People Also Ask
- How can you use lidocaine patches for multiple sore spots? A Guide to Safe, Effective Pain Relief
- How does the lidocaine patch work? Targeted Relief for Nerve Pain Explained
- What systemic side effects can lidocaine patches cause? Minimizing Risks for Safe Pain Relief
- Is it safe to use lidocaine patches while breastfeeding? Expert Guidance for Nursing Mothers
- How is the lidocaine patch administered? A Step-by-Step Guide for Safe & Effective Pain Relief
















