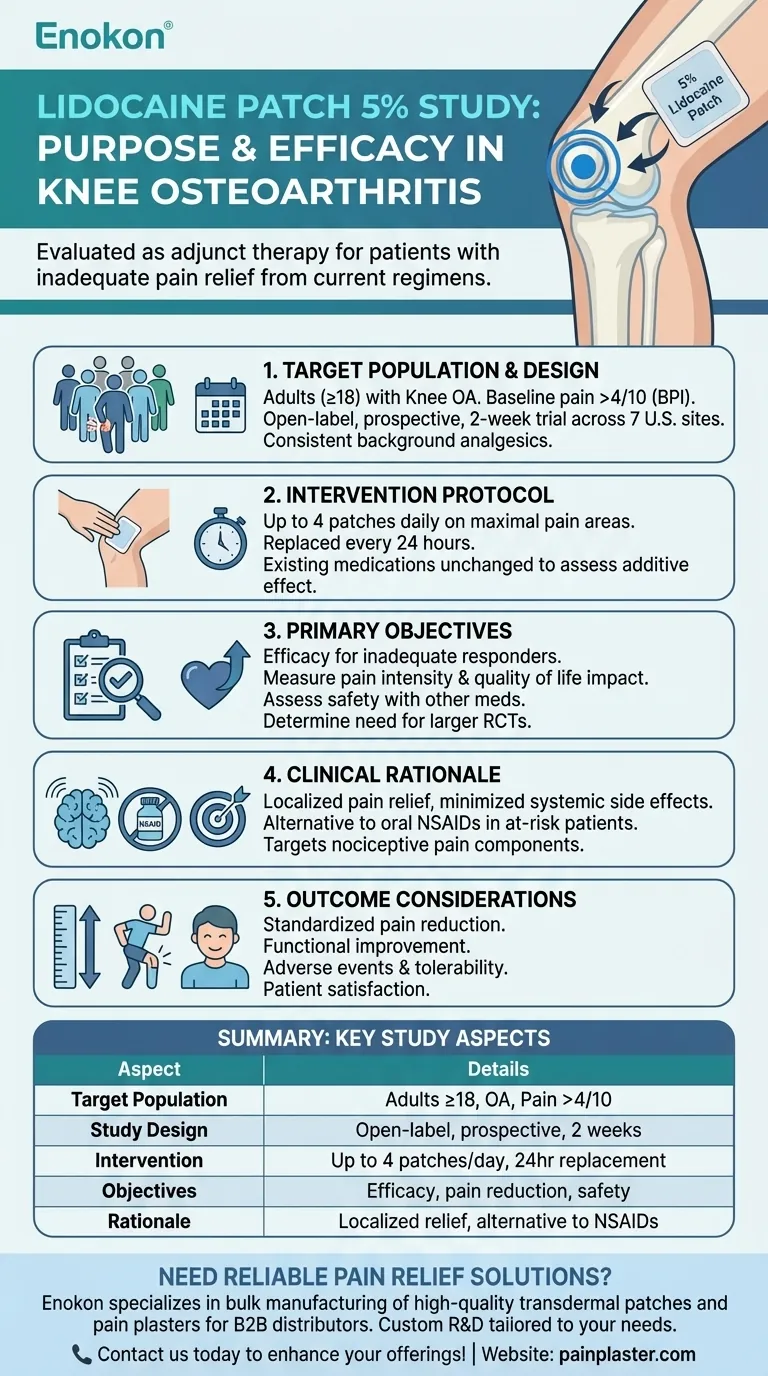
The purpose of the lidocaine patch 5 percent study in osteoarthritis patients was to evaluate its effectiveness as an adjunct therapy for knee osteoarthritis pain in individuals who had not achieved adequate relief from their current analgesic regimens. This open-label, non-randomized trial sought to determine if the patch could provide meaningful pain reduction and whether larger controlled studies were justified. The study focused on patients with moderate-to-severe pain, maintaining their existing medications while adding the patch to target localized knee pain.

Key Points Explained:
-
Target Population and Study Design
- Included adults (≥18 years) with osteoarthritis in one or both knees
- Required baseline pain intensity >4/10 on the Brief Pain Inventory
- Open-label, prospective design across 7 U.S. sites
- 2-week evaluation period with consistent background analgesics
-
Intervention Protocol
- Patients applied up to 4 patches daily to areas of maximal knee pain
- Patches were replaced every 24 hours
- Existing pain medications remained unchanged during the study
- Allowed assessment of the patch's additive effect to standard therapy
-
Primary Objectives
- Evaluate efficacy in patients with inadequate response to current analgesics
- Measure impact on pain intensity and quality of life
- Assess safety profile when combined with other pain medications
- Determine need for future randomized controlled trials
-
Clinical Rationale
- Addresses localized pain while minimizing systemic side effects
- Potential alternative to oral NSAIDs in patients with comorbidities
- Targets nociceptive pain components in osteoarthritis
- Builds on prior evidence from neuropathic pain studies
-
Outcome Considerations
- Pain reduction measured through standardized scales
- Functional improvement assessments
- Documentation of adverse events and tolerability
- Patient-reported satisfaction with therapy
The study's findings could influence clinical decisions about multimodal pain management strategies for osteoarthritis, particularly for patients requiring additional localized therapy beyond oral medications. Its design reflects real-world application scenarios where the patch would be used alongside existing treatments rather than as monotherapy.
Summary Table:
| Aspect | Details |
|---|---|
| Target Population | Adults (≥18 years) with osteoarthritis in one or both knees, pain >4/10 |
| Study Design | Open-label, prospective, 7 U.S. sites, 2-week evaluation |
| Intervention | Up to 4 patches daily on maximal knee pain, replaced every 24 hours |
| Primary Objectives | Efficacy in inadequate responders, pain reduction, safety with other meds |
| Clinical Rationale | Localized pain relief, alternative to oral NSAIDs, targets nociceptive pain |
| Outcome Measures | Pain reduction, functional improvement, adverse events, patient satisfaction |
Need reliable pain relief solutions for osteoarthritis patients?
At Enokon, we specialize in bulk manufacturing of high-quality transdermal patches and pain plasters for healthcare and pharma distributors. Our technical expertise ensures custom R&D and development tailored to your needs.
📞 Contact us today to discuss how our products can enhance your pain management offerings!
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How are lidocaine patches typically used for pain relief during pregnancy? A Guide to Safe, Targeted Relief
- How does the lidocaine patch work? Targeted Relief for Nerve Pain Explained
- How is the lidocaine patch administered? A Step-by-Step Guide for Safe & Effective Pain Relief
- When should someone contact a doctor regarding lidocaine patch use? Ensure Safe Pain Relief
- Is it safe to use lidocaine patches while breastfeeding? Expert Guidance for Nursing Mothers
















